To use all functions of this page, please activate cookies in your browser.
my.chemeurope.com
With an accout for my.chemeurope.com you can always see everything at a glance – and you can configure your own website and individual newsletter.
- My watch list
- My saved searches
- My saved topics
- My newsletter
Dark stateIn quantum mechanics a physical system, like an atom or molecule, is described by a quantum state, different states can have different energy and a system can make a transition from one energy level to another by emitting or absorbing a photon. However, not all transitions between arbitrary states are allowed. A state that can not be accessed by absorbing a photon is called a dark state. In an experiment using laser light to induce transitions between energy levels, atoms can spontaneously decay into a state that is not coupled to any other level by the laser light, atoms in this dark state no longer absorb or emit photons and therefore appear dark. Product highlightIn PracticeExperiments in atomic physics are often done with a laser of a specific frequency ω (meaning the photons have a specific energy), so they only couple one set of states with a particular energy E1 to another set of states with an energy In theoryWhether or not we say a transition between a state |1> and a state |2> is allowed often depends on how detailed the model is that we use for the atom-light interaction. From a particular model follow a set of selection rules that determine which transitions are allowed and which are not. Often these selection rules can be boiled down to conservation of angular momentum (the photon has angular momentum). In most cases we only consider an atom interacting with the electric dipole field of the photon. Then some transitions are not allowed at all, others are only allowed for photons of a certain polarization. Let's consider for example the hydrogen atom. The transition from the state 12S1 / 2 with mj=-1/2 tot the state 22P3 / 2 with mj=-1/2is only allowed for light with polarization along the z axis (quantization axis) of the atom. The state 22P3 / 2 with mj=-1/2 therefore appears dark for light of other polarizations. Transitions from the 2S level to the 1S level are not allowed at all. The 2S state can not decay to the ground state by emitting a single photon. It can only decay by collisions with other atoms or by emitting multiple photons. Since these events are rare, the atom can remain in this excited state for a very long time, such an excited state is called a metastable state. External links+A description with animations and pictures from Univ. of Bonn |
| This article is licensed under the GNU Free Documentation License. It uses material from the Wikipedia article "Dark_state". A list of authors is available in Wikipedia. |





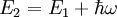 . The atom can however still decay spontaneously into a third state by emitting a photon of a different frequency. The new state with energy
. The atom can however still decay spontaneously into a third state by emitting a photon of a different frequency. The new state with energy 

