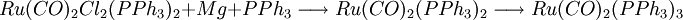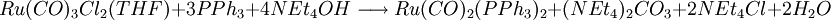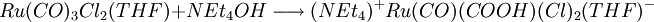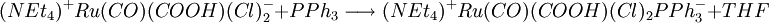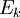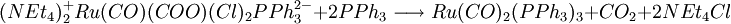To use all functions of this page, please activate cookies in your browser.
my.chemeurope.com
With an accout for my.chemeurope.com you can always see everything at a glance – and you can configure your own website and individual newsletter.
- My watch list
- My saved searches
- My saved topics
- My newsletter
Dicarbonyltris(triphenylphosphine)ruthenium (0)Dicarbonyltris(triphenylphosphine)ruthenium (0) or Roper's complex is a ruthenium metal carbonyl . In Ru(CO)2(PPh3)3 carbon monoxide ligands and three triphenylphosphine ligands are coordinated to a central ruthenium atom. This compound loses a phosphine ligand very easily can replace it alkynes or olefins. The related organometallic complex Ru(CO)2H2(PPh3)2 can be obtained by exposing the complex to hydrogen gas and is a catalyst in the Murai olefin coupling reaction between terminal alkenes and the ortho C-H position of a phenone. Product highlight
PreparationThe compound can be prepared by magnesium reduction in the presence of a large excess of phosphine. The 16-electron intermediate can actually be isolated.
A recent base promoted method involves the reduction of bivalent ruthenium in tricarbonyldichloro ruthenium (II) with triphenylphosphine and the ammonium salt tetraethylammonium hydroxide . The overall reaction for this one-pot synthesis is: but the reactants are added sequentially. The first step is the formation of a hydroxyl - carbonyl adduct to an anion: next solvatated tetrahydrofuran is replaced by phosphine: next the carboxylic acid ligand is deprotonated by hydroxide forming the dianion and water in this compound carbon monoxide is effectively oxidized to carbon dioxide with the reduction of Ru (II) to Ru(0) and in a dissociative chemical reaction carbon dioxide is removed. The two remaining chlorine ligands are replaced by two more phosphine groups in the final step. The generated carbon dioxide is trapped: In this sequence low reaction temperatures and the order in which the reagents are added are important. Ru(CO)2(PPh3)3 is air stable when dry but in THF conversion occurs to Ru(CO)2(PPh3)3O2. The compound has a trigonal bipyramidal molecular geometry and exists as a mixture of two isomers that rapidly interconvert in solution. References
Categories: Ruthenium compounds | Carbonyl complexes |
| This article is licensed under the GNU Free Documentation License. It uses material from the Wikipedia article "Dicarbonyltris(triphenylphosphine)ruthenium_(0)". A list of authors is available in Wikipedia. |