To use all functions of this page, please activate cookies in your browser.
my.chemeurope.com
With an accout for my.chemeurope.com you can always see everything at a glance – and you can configure your own website and individual newsletter.
- My watch list
- My saved searches
- My saved topics
- My newsletter
Dispersion (water waves)Dispersion of water waves generally refers to frequency dispersion. That is, water, in fluid dynamics, is generally considered to be a dispersive medium; which means that the velocity of the wave front travels as a function of frequency so that spatial and temporal phase properties of the wave propagation are constantly changing. So, for example, waves travelling in water with a longer wavelength and period travel faster than those with a shorter wavelength and period. This phenomenon is expressed by the so-called linear dispersion relationship, which may be given as,
Product highlightHere ω is the angular frequency, g is gravity, k is the wavenumber, and h is the height. The height here refers to the surface wave phenomena and is incorporated into the given form of the dispersion relation. The hyperbolic tangent is meant to physically model the observation that dispersion asymptotically goes to zero as h gets small. Under these assumptions, the linear wave celerity (scalar valued velocity) may then be expressed as,
These equations are correct to the second order perturbation theory expansion. To the third order, and for deep water, the dispersion relation is,
where A is wave amplitude. This implies that large waves travel faster than small ones of the same frequency. This is only noticeable when the wave amplitude is large. Historically, this equation This equation is quite frequently approximated to
See also
|
| This article is licensed under the GNU Free Documentation License. It uses material from the Wikipedia article "Dispersion_(water_waves)". A list of authors is available in Wikipedia. |


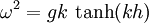 .
.



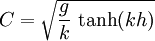 .
.
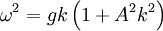 .
.
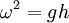 , which was derived by Joseph Louis Lagrange.
, which was derived by Joseph Louis Lagrange.


