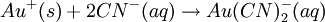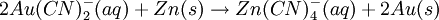To use all functions of this page, please activate cookies in your browser.
my.chemeurope.com
With an accout for my.chemeurope.com you can always see everything at a glance – and you can configure your own website and individual newsletter.
- My watch list
- My saved searches
- My saved topics
- My newsletter
Heap leachingHeap leaching is an industrial mining process to extract precious metals and copper compounds from ore. Product highlight
ProcessThe mined ore is crushed into small chunks and heaped on an impermeable plastic and/or clay lined leach pad where it can be irrigated with a leach solution to dissolve the valuable metals. Either sprinklers, or often drip irrigation, is used to minimize evaporation. The solution then percolates through the heap and leaches out the precious metal. This can take several weeks. The leach solution containing the dissolved metals is then collected. Precious metals methodThe crushed ore is irrigated with a dilute cyanide solution. The solution percolates through the heap and leaches out the precious metal. This can take several weeks. The solution containing the precious metals ("pregnant solution") continues percolating through the crushed ore until it reaches the liner at the bottom of the heap where it drains into a storage (pregnant solution) pond. After separating the precious metals from the pregnant solution, the dilute cyanide solution (now called "barren solution") is normally re-used in the heap-leach-process or occasionally sent to an industrial water treatment facility where the residual cyanide is treated and residual metals are removed. The water is then discharged to the environment, posing possible water pollution. During the extraction phase, the gold ions form complex ions with the cyanide:
Recuperation of the gold is readily achieved with a redox-reaction:
Copper methodThe method is essentially similar to the Cyanide method, above, except sulphuric acid is used to dissolve copper from its ores. The acid is recycled from the solvent extraction circuit (see solvent extraction-electrowinning under mineral processing) and reused on the leach pad. A particular byproduct is iron(II) sulfate, jarosite, which is produced as a byproduct of leaching pyrite, and sometimes even the same sulphuric acid that is needed for the process. Although the heap leaching is a low cost process, it normally has recovery rates of 60-70%, although there are exceptions. It is normally most profitable when used on low-grade ores. (Higher-grade ores are usually put through more complex milling processes where higher grades x higher recoveries = higher profits. The actual process depends on the metallurgical properties of the ore.) Sulphuric acid heap leaching of nickelThe method is an acid heap leaching method like that of the copper method in that it utilises sulphuric acid instead of cyanide solution to dissolve the target minerals from crushed ore. The method has been developed by European Nickel PLC for the rock laterite deposits of Turkey and the Balkans.
See also |
|
| This article is licensed under the GNU Free Documentation License. It uses material from the Wikipedia article "Heap_leaching". A list of authors is available in Wikipedia. |