To use all functions of this page, please activate cookies in your browser.
my.chemeurope.com
With an accout for my.chemeurope.com you can always see everything at a glance – and you can configure your own website and individual newsletter.
- My watch list
- My saved searches
- My saved topics
- My newsletter
Ferrocyanide
Ferrocyanide is the name of the anion Fe(CN)64−. In aqueous solutions, this coordination complex is relatively unreactive. It is usually available as the potassium salt potassium ferrocyanide, which has the formula K4Fe(CN)6. Product highlight
Coordination chemistry[Fe(CN)6]4− is a diamagnetic species, featuring low-spin iron(II) center in an octahedral ligand environment. Although many salts of cyanide are highly toxic, ferro- and ferricyanides are less toxic because they tend not to release free cyanide.[1] Its most important reaction is its oxidation to ferricyanide:
This conversion can be followed spectroscopically at 535 nm with an absorption coefficient of 21600 M−1 cm−1. Treatment of ferrocyanide with ferric-containing compounds affords Prussian Blue, an intensely blue polymer that is widely used as a pigment.[1] Use in biochemistryFerrocyanide and its oxidized product ferricyanide, [Fe(CN)6]3−, are impermeable to the plasma membrane. For this reason ferrocyanide has been used as a probe of extracellular electron receptor in the study of redox reactions in cells. Ferricyanide is used thus any increase in ferrocyanide can be attributed to secretions of reductants or "Trans Plasma Membrane Electron Transport" (TPMET) activity. Potassium ferricyanide is often used as a mediator in the test strips used with blood glucose meters by people suffering from diabetes. It is used in this application because it is easily reduced to potassium ferrocyanide. NomenclatureAccording to the recommendations of IUPAC, ferrocyanide should be called "hexacyanoferrate(II)." References
Categories: Cyanides | Iron compounds | Anions | Coordination compounds |
|
| This article is licensed under the GNU Free Documentation License. It uses material from the Wikipedia article "Ferrocyanide". A list of authors is available in Wikipedia. |





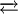 [Fe(CN)6]3− + e−
[Fe(CN)6]3− + e−


