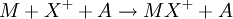To use all functions of this page, please activate cookies in your browser.
my.chemeurope.com
With an accout for my.chemeurope.com you can always see everything at a glance – and you can configure your own website and individual newsletter.
- My watch list
- My saved searches
- My saved topics
- My newsletter
Ion attachment mass spectrometry
Ion-attachment mass spectrometry (IAMS) is a form of mass spectrometry that uses a "soft" form of ionization similar to chemical ionization in which a cation is attached to the analyte molecule in a reactive collision: Where M is the analyte molecule, X+ is the cation and A is a non-reacting collision partner.[1] Product highlight
PrincipleThis technique is applicable to gases or any materials that can be vaporized. It uses a non-fragmenting non-conventional ionisation mode, by attachment of a lithium (or alkaline) ion to the gas to be analysed with a more traditional mass filter. This instrument is more dedicated to analysis of moderately-sized molecules such as organic or aromatic compounds.[2] ApplicationsCurrently, it is used industrially to verify, with a high throughput, the concentrations of brominated flame retardants (BFR) in plastics in compliance with European RoHS (Restriction of Hazardous Substances) regulation in place since 2006. The banned molecules include PBB and PBDE, whose concentration should not exceed 0.1% w/w. [3][4][5] IAMS has also been used to analyze diesel exhaust particles[6], in ceramic processing [7] and in semiconductor critical SiO2 etch processing. References
Bibliography
|
|||||||
| This article is licensed under the GNU Free Documentation License. It uses material from the Wikipedia article "Ion_attachment_mass_spectrometry". A list of authors is available in Wikipedia. | |||||||