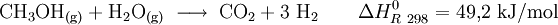To use all functions of this page, please activate cookies in your browser.
my.chemeurope.com
With an accout for my.chemeurope.com you can always see everything at a glance – and you can configure your own website and individual newsletter.
- My watch list
- My saved searches
- My saved topics
- My newsletter
Methanol reformerA methanol refomer is a device used in chemical engineering, especially in the area of fuel cell technology, which can produce pure hydrogen gas and carbon dioxide by reacting a methanol and water (steam) mixture. Product highlightMethanol is transformed into hydrogen and carbon dioxide by pressure and heat and interaction with a catalyst. TechnologyA mixture of water and methanol with a molar concentration ratio (water:methanol) of 1.3 - 1.5 is pressurized to approximately 20 bar, vaporized and heated to a temperature of 250 - 280 °C. The hydrogen that is created is separated through the use of a hydrogen-permeable membrane made of a palladium and silver alloy. There are two basic methods of conducting this process.
With either design, not all of the hydrogen is removed from the product of the reaction, so the remaining gas mixture still contains a significant amount of hydrogen. As such, this resulting mixture is often mixed with air and burned. The heat energy produced by burning this mixture can be used for heating purposes. Advantages and disadvantagesMethanol reformers are being considered as a component of a hydrogen fuel cell-powered vehicle. A prototype car, the NECAR 5, was introduced by Daimler-Chrysler in the year 2000. The primary advantage of a vehicle with a reformer is that it does not need a pressurized gas tank to store hydrogen fuel; instead methanol is stored as a liquid. This could help ease the public's concern over the danger of hydrogen and thereby make fuel cell powered vehicles more attractive. However, methanol, like gasoline, is toxic and extremely flammable. The cost of the PdAg membrane and its susceptibility to damage by temperature changes provide obstacles to adoption. Another problem is that although hydrogen power produces energy without CO2, a methanol reformer creates the gas as a byproduct. The high level of greenhouse gases in our atmosphere significantly contributes to global warming. References
Categories: Hydrogen production | Fuel cells |
| This article is licensed under the GNU Free Documentation License. It uses material from the Wikipedia article "Methanol_reformer". A list of authors is available in Wikipedia. |