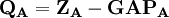To use all functions of this page, please activate cookies in your browser.
my.chemeurope.com
With an accout for my.chemeurope.com you can always see everything at a glance – and you can configure your own website and individual newsletter.
- My watch list
- My saved searches
- My saved topics
- My newsletter
Mulliken population analysisMulliken charges arise from the Mulliken population analysis and provide a means of estimating partial atomic charges from calculations carried out by the methods of computational chemistry, particularly those based on the linear combination of atomic orbitals molecular orbital method. If the coefficients of the basis functions in the molecular orbital are Cμi for the μ'th basis function in the i'th molecular orbital, the density matrix terms are: Product highlightfor a closed shell system where each molecular orbital is doubly occupied. The population matrix
The The problem with this approach is the equal division of the off-diagonal terms between the two basis functions. This leads to charge separations in molecules that are exaggerated. Several other methods have used to estimate atomic charges in molecules. See alsoReferences
|
| This article is licensed under the GNU Free Documentation License. It uses material from the Wikipedia article "Mulliken_population_analysis". A list of authors is available in Wikipedia. |


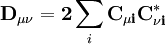



 then has terms
then has terms
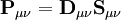
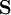 is the overlap matrix of the basis functions. The sum of all terms of
is the overlap matrix of the basis functions. The sum of all terms of 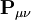 is N - the total number of electrons. The Mulliken population analysis aims first to divide N among all the basis functions. This is done by taking the diagonal element of
is N - the total number of electrons. The Mulliken population analysis aims first to divide N among all the basis functions. This is done by taking the diagonal element of 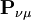 , this simplifies to just the sum of a row. This defines the gross orbital population (GOP) as
, this simplifies to just the sum of a row. This defines the gross orbital population (GOP) as
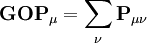
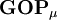 terms sum to N and thus divide the total number of electrons between the basis functions. It remains to sum these terms over all basis functions on a given atom A to give the gross atom population (GAP). The sum of
terms sum to N and thus divide the total number of electrons between the basis functions. It remains to sum these terms over all basis functions on a given atom A to give the gross atom population (GAP). The sum of 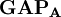 terms is also N. The charge,
terms is also N. The charge, 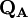 , is then defined as the difference between the number of electrons on the isolated free atom, which is the atomic number
, is then defined as the difference between the number of electrons on the isolated free atom, which is the atomic number 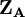 , and the gross atom population:
, and the gross atom population: