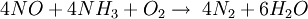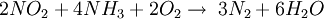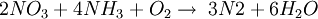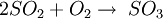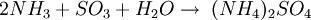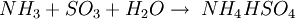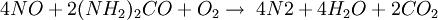To use all functions of this page, please activate cookies in your browser.
my.chemeurope.com
With an accout for my.chemeurope.com you can always see everything at a glance – and you can configure your own website and individual newsletter.
- My watch list
- My saved searches
- My saved topics
- My newsletter
Selective catalytic reductionSelective catalytic reduction (SCR) is a means of converting nitrogen oxides, also referred to as NOx with the aid of a catalyst into diatomic nitrogen, N2, and water, H2O. A gaseous reductant, typically anhydrous ammonia, aqueous ammonia or urea, is added to a stream of flue or exhaust gas and is absorbed onto a catalyst. Carbon dioxide, CO2 is a reaction product when urea is used as the reductant. Selective catalytic reduction of NOx using ammonia as the reducing agent was patented in the United States by the Englehard Corporation in 1957. Development of SCR technology continued in Japan and the US in the early 1960’s with research focusing on less expensive and more durable catalyst agents. The first large scale SCR was installed by the IHI Corporation in 1978. [1] Commercial selective catalytic reduction systems have typically found on large utility boilers, industrial boilers, and municipal should waste boilers and have been shown to reduce NOx from 70-95%.[2] More recent applications include large diesel engines, such as those found on large ships, diesel locomotives, combustion turbines, and even automobiles. Product highlight
The ReactionThe NOX reduction reaction takes place as the gases passes through the catalyst chamber. Before entering the catalyst the ammonia, or other reductant, is injected and mixed with the gases. The chemical equation for a stoichiometric reaction using either anhydrous or aqueous ammonia for a selective catalytic reduction process is as follows: With several secondary reactions The reaction for urea instead of either anhydrous or aqueous ammonia is as follows: The ideal reaction has an optimal temperature range between 675 and 840°F, but can operate from 450 to 840°F with longer residence times needed. The minimum temperature is effected by the various fuels, gas constituents and catalyst geometry. Other possible reductants include cyanuric acid and ammonium sulfate [3] CatalystsSCR catalysts are manufactured from various ceramic materials used as a carrier, such as titanium oxide, and active catalytic components are usually either oxides of base metals (such as vanadium and tungsten), zeolites, and various precious metals. All catalyst components have their own unique advantages and disadvantages. Base metal catalysts, such as the vanadium and tungsten, lack high thermal durability, but are less expensive and operate very well at the temperature ranges most commonly seen in industrial and utility boiler applications. Thermal durability is particularly important for automotive SCR applications that incorporate the use of a diesel particulate filter with forced regeneration. They also have a high catalyzing potential to oxidize SO2 into SO3, which can be extremely damaging to its acidic properties. [4] Zeolite catalysts have the potential to operate at significantly higher temperatures than base metal catalysts, with the ability to withstand long term operational temperatures of 1200°F, and transient conditions of up to 1560°F. Zeolite also have a lower potential for potentially damaging SO2 oxidation. [4] Recently developed iron and copper exchanged zeolite urea SCRs have been developed with approximately equal performance to that of vanadium urea-SCRs if the fraction of the NO2 is 20% to 50% of the total NO_X.[5] The two most common designs of SCR catalyst geometry used today are honeycomb and plate. The honeycomb form usually is an extruded ceramic applied homogeneously throughout the ceramic carrier or coated on the substrate. Like the various types of catalysts, their configuration also has advantages and disadvantages. Plate type catalysts have lower pressure drops and are less susceptible to plugging and fouling than the honeycomb types, however plate type configurations are significantly larger and more expensive. Honeycomb configurations are significantly smaller than plate types, but have higher pressure drops and plug much more easily.[6] ReductantsSeveral reductant are currently used SCR applications including anhydrous ammonia, aqueous ammonia or urea. All three reductant are widely available in large quantities. Pure anhydrous ammonia while extremely toxic and difficult to safely store, need no further conversion to operate within an SCR. Since it requires no further conversion to be useful, it is typically favored by large industrial SCR operators. Aqueous ammonia must be hydrolized in order to be used but it is significantly safer to store and transport than anhydrous ammonia. Urea is the safest to store, but requires conversion to ammonia through thermal decomposition in order to be used as an effective reductant. [7] Technical problems with automotive SCR unitsIn order to ensure that the SCR unit remains free from contaminants, correct materials of construction must be used for both storage and dispensing. Manufacturers of the SCR unit have specified that, without using compatible materials of construction, ions can be passed from the dispensing materials into the porous head on the SCR unit. This can render the SCR unit ineffective and reduce its life expectancy by more than 60%. The biggest issue with SCR is the necessity to tune the SCR system to the engine operating cycle. This requires running the engine through a simulation of the operating cycle of the machine it will be fitted to. The simulation can be run on a dynamometer, or on an actual piece of equipment during its normal work day (data logging). Even at best, data logging tends to be inaccurate, as no two operators will use the equipment in the same way. Even when used for the same general purposes (i.e., a truck delivering goods to stores in a city), small differences in the route such as hills, one-way streets, amount unloaded, etc., can make the engine loads different enough that effectiveness of the system will suffer. Another common problem with all SCR systems is the release of unreacted ammonia referred to as ammonia slip. Slip can occur when catalyst temperatures are not in the optimal range for the reaction or when too much ammonia is injected into the process. Power plantsIn power stations, the same basic technology is employed for removal of NOx from the flue gas of boilers used in power generation and industry. The SCR unit is generally located between the furnace economizer and the air heater and the ammonia is injected into the catalyst chamber through an ammonia injection grid. As in other SCR applications, the temperature of operation is critical. Ammonia slip is also an issue with SCR technology used in power plants. Other issues which must be considered in using SCR for NOx control in power plants are the formation of ammonium sulfate and ammonium bisulfate due to the sulfur content of the fuel as well as the undesirable catalyst-caused formation of SO3 from the SO2 and O2 in the flue gas. A further operational difficulty in coal-fired boilers is the blinding of the catalyst by fly ash from the fuel combustion. This requires the usage of sootblowers, sonic horns and careful design of the ductwork and catalyst materials to avoid plugging by the fly ash. See also
References
|
|
| This article is licensed under the GNU Free Documentation License. It uses material from the Wikipedia article "Selective_catalytic_reduction". A list of authors is available in Wikipedia. |