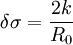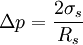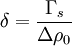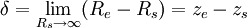To use all functions of this page, please activate cookies in your browser.
my.chemeurope.com
With an accout for my.chemeurope.com you can always see everything at a glance – and you can configure your own website and individual newsletter.
- My watch list
- My saved searches
- My saved topics
- My newsletter
Tolman lengthThe Tolman length δ (also known as Tolman's delta) measures the extent by which the surface tension of a small liquid drop deviates from its planar value. It is conveniently defined in terms of an expansion in 1 / R, with R = Re the equimolar radius of the liquid drop, of the pressure difference across the droplet's surface: Product highlight
In this expression, Δp = pl − pv is the pressure difference between the (bulk) pressure of the liquid inside and the pressure of the vapour outside, and σ is the surface tension of the planar interface, i.e. the interface with zero curvature Another way to define the tolman length is to consider the radius dependence of the surface tension, σ(R). To leading order in 1 / R one has:
Here σ(R) denotes the surface tension (or (excess) surface free energy) of a liquid drop with radius R, whereas σ denotes its value in the planar limit. In both definitions (1) and (2) the Tolman length is defined as a coefficient in an expansion in 1 / R and therefore does not depend on R. Furthermore, the Tolman length can be related to the radius of spontaneous curvature when one compares the free energy method of Helfrich with the method of Tolman:
Any result for the Tolman length therefore gives information about the radius of spontaneous curvature, R0. If the Tolman length is known to be positive (with k > 0) the interface tends to curve towards the liquid phase, whereas a negative Tolman length implies a negative R0 and a preferred curvature towards the vapour phase. Apart from being related to the radius of spontaneous curvature, the Tolman length can also be linked to the surface of tension'. The surface of tension, positioned at R = Rs, is defined as the surface for which the Laplace equation holds exactly for all droplet radii:
where σs = σ(R = Rs) is the surface tension at the surface of tension. Using the Gibbs adsorption equation, Tolman himself showed that the Tolman length can be expressed in terms of the adsorbed amount at the surface of tension at coexistence
where Δρ0 = ρl,0 − ρv,0; the subscript zero to the density denotes the value at two-phase coexistence. It can be shown that the difference between the location of the surface of tension and of the equimolar dividing surface proposed by Gibbs yields the value of the Tolman length:
where the ze,zs denote the locations of the corresponding surfaces making the magnitude of the Tolman length in the order of nanometers. References
Categories: Physical chemistry | Thermodynamics |
| This article is licensed under the GNU Free Documentation License. It uses material from the Wikipedia article "Tolman_length". A list of authors is available in Wikipedia. |





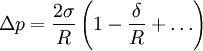 (1)
(1)
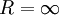 . The Tolman length
. The Tolman length 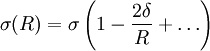 (2)
(2)