To use all functions of this page, please activate cookies in your browser.
my.chemeurope.com
With an accout for my.chemeurope.com you can always see everything at a glance – and you can configure your own website and individual newsletter.
- My watch list
- My saved searches
- My saved topics
- My newsletter
Curie constantCurie's constant is Additional recommended knowledge
where N is the number of magnetic moments, μ is an individual magnetic moment and kB is Boltzmann's constant. It comes from Curie's Law, which is the relationship between magnetic susceptibility and temperature of a paramagnetic material
This equation was first derived by Pierre Curie (Marie Curie's husband.) Because of the relationship between magnetic susceptibility χ, magnetization M and applied magnetic field H:
this shows that for a paramagnetic system of non-interacting magnetic moments, magnetization M is inversely related to temperature T (see Curie's Law). See also |
| This article is licensed under the GNU Free Documentation License. It uses material from the Wikipedia article "Curie_constant". A list of authors is available in Wikipedia. |





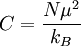 ,
,
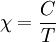 .
.
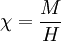 ,
,


