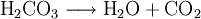To use all functions of this page, please activate cookies in your browser.
my.chemeurope.com
With an accout for my.chemeurope.com you can always see everything at a glance – and you can configure your own website and individual newsletter.
- My watch list
- My saved searches
- My saved topics
- My newsletter
EffervescenceLook up effervescence in Wiktionary, the free dictionary.
Effervescence is the escape of gas from an aqueous solution. The term is used to describe the foaming or fizzing that results from a release of gas. In the lab, a common example of effervescence is the addition of hydrochloric acid to a block of limestone. If a few pieces of marble or an antacid tablet are put in hydrochloric acid in a test tube fitted with a cork, effervescence of carbon dioxide can be witnessed. Additional recommended knowledgeThis process is generally represented by the following reaction, where a pressurized dilute solution of carbonic acid in water releases gaseous carbon dioxide at decompression:
In simple terms, it is the result of the chemical reaction occurring in the liquid which produces a gaseous product. See also |
| This article is licensed under the GNU Free Documentation License. It uses material from the Wikipedia article "Effervescence". A list of authors is available in Wikipedia. |