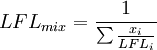To use all functions of this page, please activate cookies in your browser.
my.chemeurope.com
With an accout for my.chemeurope.com you can always see everything at a glance – and you can configure your own website and individual newsletter.
- My watch list
- My saved searches
- My saved topics
- My newsletter
Flammability limit
Flammability limits, also called flammable limits, give the proportion of combustible gases in a mixture, between which limits this mixture is flammable. Gas mixtures consisting of combustible, oxidizing, and inert gases are only flammable under certain conditions. The lower flammable limit (LFL) describes the leanest mixture that is still flammable, i.e. the mixture with the smallest fraction of combustible gas, while the upper flammable limit (UFL) gives the richest flammable mixture. Increasing the fraction of inert gases in a mixture raises the LFL and decreases UFL. Additional recommended knowledgeFlammability limits of mixtures of several combustible gases can be calculated using Le Chatelier's mixing rule for combustible volume fractions xi:
and similar for UFLmix. Temperature and pressure also influences flammability limits. Higher temperature results in lower LFL and higher UFL, while greater pressure increases both values. The effect of pressure is very small at pressures below 10 millibar and difficult to predict, since it has hardly been studied. See alsoCategories: Chemical processes | Thermodynamics | Chemical reactions |
||
| This article is licensed under the GNU Free Documentation License. It uses material from the Wikipedia article "Flammability_limit". A list of authors is available in Wikipedia. |