To use all functions of this page, please activate cookies in your browser.
my.chemeurope.com
With an accout for my.chemeurope.com you can always see everything at a glance – and you can configure your own website and individual newsletter.
- My watch list
- My saved searches
- My saved topics
- My newsletter
Peierls StressPeierls stress is the force first discovered by Rudolph Peierls and modified by David Nabarro needed to move a dislocation within a plane of atoms in the unit cell. The magnitude varies periodically as the dislocation moves within the plane. Peierls stress depends on the size and width of a dislocation and the distance between planes. Because of this, Peierls stress decreases with increasing distance between atomic planes. Yet since the distance between planes increases with planar density, slip of the dislocation is preferred on closely packed planes. Product highlight
Peierls-Nabarro Stress Proportionality:
The Peierls Stress and Yield Strength Temperature SensitivityThe Peierls Stress also relates to the temperature sensitivity of the yield strength of a material because it too depends on both short range atomic order and atomic bond strength. As temperature increases, the vibration of atoms increases and thus both peierls stress and yield strength decrease as a result of weaker atomic bond strength at high temperatures. References
|
| This article is licensed under the GNU Free Documentation License. It uses material from the Wikipedia article "Peierls_Stress". A list of authors is available in Wikipedia. |





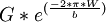
 the dislocation width
the dislocation width


