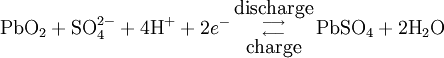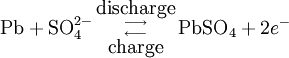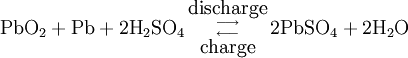To use all functions of this page, please activate cookies in your browser.
my.chemeurope.com
With an accout for my.chemeurope.com you can always see everything at a glance – and you can configure your own website and individual newsletter.
- My watch list
- My saved searches
- My saved topics
- My newsletter
Rechargeable batteryA rechargeable battery, also known as a storage battery, is a group of two or more secondary cells. These batteries can be restored to full charge by the application of electrical energy. In other words, they are electrochemical cells in which the electrochemical reaction that releases energy is readily reversible. Rechargeable electrochemical cells are therefore a type of accumulator. They come in many different designs using different chemicals. Commonly used secondary cell chemistries are lead and sulfuric acid, nickel cadmium (NiCd), nickel metal hydride (NiMH), lithium ion (Li-ion), and lithium ion polymer (Li-ion polymer). Rechargeable batteries can offer an economic benefit when used instead of one-time-use disposable batteries. Most rechargeable battery technology has been adapted into the standard “AA,” “AAA,” “C,” “sub-C,” “D,” and “9-volt” configurations that consumers are familiar with. While the rechargeable versions of these types of cells have a higher up-front cost than disposable batteries, rechargeable batteries can be discharged and recharged many times. Some manufacturers of NiMH type rechargeable batteries claim a lifespan up to 3000 charge cycles for their batteries. Additional recommended knowledge
Usage and applicationsUnlike nonrechargeable batteries (primary cells), secondary cells must be charged before use. Attempting to recharge nonrechargeable batteries is not advised as it has a small chance of causing a battery explosion. Some types of rechargeable batteries are susceptible to damage due to reverse charging if they are fully discharged; other types need to be fully discharged occasionally in order to maintain the capacity for deep discharge. Fully integrated battery chargers that optimize the charging current are available. Rechargeable batteries currently are used for lower power applications such as automobile starters, portable consumer devices, tools, and uninterruptible power supplies. Emerging applications in hybrid vehicles and electric vehicles are driving the technology to improve cost, reduce weight, and increase lifetime. Future applications are proposed to use rechargeable batteries for load leveling, where they would store baseline electric power for use during peak load periods, and for renewable energy uses, such as storing power generated from photovoltaic arrays during the day to be used at night. The National Electrical Manufacturers Association has estimated that U.S. demand for rechargeables is growing twice as fast as demand for nonrechargeables.[1] Charging
During charging, the positive active material is oxidized, producing electrons, and the negative material is reduced, consuming electrons. These electrons constitute the current flow in the external circuit. The electrolyte may serve as a simple buffer for ion flow between the electrodes, as in lithium-ion and nickel-cadmium cells, or it may be an active participant in the electrochemical reaction, as in lead-acid cells. The reactions in lead-acid cells are illustrated in the following diagrams.
The half-cell reactions and overall cell reaction for the lead-acid system are as follows: Positive electrode
Negative electrode
Overall reaction
The energy used to charge rechargeable batteries mostly comes from mains electricity using an adapter unit. It can be wired or wireless[citation needed]. Charging backup batteries using off-peak energy paid for by on-peak excess electric power from residential solar panels exactly matches the critical peak shortage and nightly electric surplus. This load-leveling function helps eliminate the need for expensive peaking power plants and helps amortize the cost of generators over more hours of operation. Charging from the 12-volt battery of a car is also possible. Human powered generators are commercially available. One can also use portable batteries to charge or to be used directly after recharging. Most battery chargers can take several hours to charge a battery (excepting Nano Titanate batteries). Most batteries can be charged in far less time than the most common simple battery chargers are capable of. Duracell and Rayovac now sell chargers that can charge AA- and AAA-size NiMH batteries in just 15 minutes; Energizer sells chargers that can additionally charge C/D-size and 9V NiMH batteries. Flow batteries don't need to be charged on place, because they can be charged by replacing the electrolyte liquid. Battery manufacturers' technical notes often refer to VPC. This is Volts Per Cell, and refers to the individual secondary cells that make up the battery. For example, to charge a 12 V battery (containing 6 cells of 2 V each) at 2.3 VPC requires a voltage of 15.6 V across the battery's terminals. Recharging electric vehiclesRecharging an electric vehicle using off-peak energy paid for by on-peak excess electric power from residential solar panels exactly matches the critical peak shortage and nightly electric surplus. While electric vehicles can charge slowly at night, raising the nightly low electric use, solar panels can lower the daytime peak, flattening the daily usage curve and lowering the cost of electric power for all users. Reverse chargingReverse charging, which damages batteries, is when a rechargeable battery is recharged with its polarity reversed. Reverse charging can occur under a number of circumstances, the two most important being:
Active ComponentsThe active components in a secondary cell are the chemicals that make up the positive and negative active materials, and the electrolyte. The positive and negative are made up of different materials, with the positive exhibiting a reduction potential and the negative having an oxidation potential. The sum of these potentials is the standard cell potential or voltage. In primary cells the positive and negative electrodes are known as the cathode and anode, respectively. Although this convention is sometimes carried through to rechargeable systems—especially with lithium-ion cells, because of their origins in primary lithium cells—this practice can lead to confusion. In rechargeable cells the positive electrode is the cathode on discharge and the anode on charge, and vice versa for the negative electrode. Example: Nickel Metal Hydride Nickel oxyhydroxide (NiOOH) is the active component in the positive, while the negative is composed of hydrogen in the form of metal hydride. The electrolyte of this secondary cell is an aqueous form of potassium hydroxide. In the discharge process, the nickel oxyhydroxide is reduced to nickel hydroxide and the metal hydride is reduced to an alloy. Nickel-Metal Hydride
Battery types
NotesFor brevity, entries in the table had to be abbreviated. For a full description, please refer to the individual article about each type. Battery types for which there is no article yet are listed below.
Less common types
Alternatives
See also
References
Categories: Electric batteries | Rechargeable batteries |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| This article is licensed under the GNU Free Documentation License. It uses material from the Wikipedia article "Rechargeable_battery". A list of authors is available in Wikipedia. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||