New record set for carbon-carbon single bond length
Advertisement
A stable organic compound has been synthesized with a record length for the bond between its carbon atoms, exceeding the assumed limit.
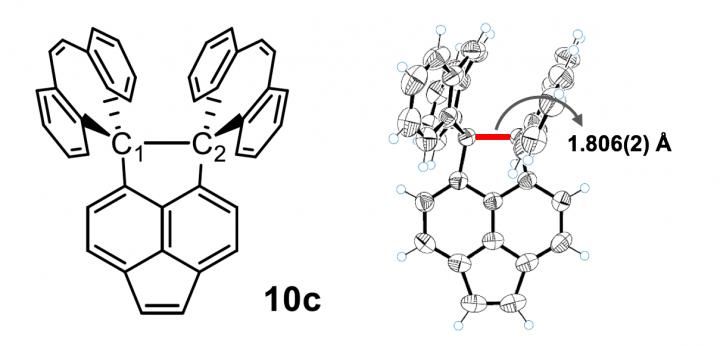
This is the chemical structure of the compound which showed the longest C-C bond.
Ishigaki Y. et al., Chem, March 8, 2018.

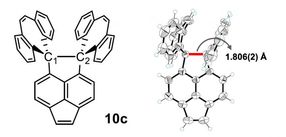
This is the 10c crystal (left) which showed the longest C-C bond and the X-ray diffraction instrument used for the analysis (right).
Hokkaido University

Hokkaido University researchers have synthesized an organic compound with a longer bond between carbon atoms than ever before - exceeding the assumed limit for carbon-carbon single bond (C-C) lengths. The researchers termed it a "hyper covalent bond."
The novel polycyclic hydrocarbon named 10c is stable, and an X-ray analysis showed that its C-C bond lengths was as long as 1.806 angstroms, longer than the previously reported world records for hydrocarbons.
Chemical bonds are formed between atoms by the transfer or sharing of electrons. Bonds become shorter the more electrons they have participating in their formation. Also, the longer the bond, the weaker it becomes. In such chemical bonds, the C-C single bond length is generally 1.54 angstroms, and altering C-C bond lengths can give organic compounds unique properties.
Previously, other researchers had estimated that the strength of C-C bonds becomes zero, making them completely unstable, when they reach a theoretical limit of 1.803 angstroms in length. But this calculation assumes a linear relationship between bond strength and length.
Yusuke Ishigaki and Takanori Suzuki of Hokkaido University and their team thought it was highly likely they could find a longer C-C bond due to evidence that the relationship between bond strength and length is actually non-linear.
By using what they call a "core-shell strategy," the team first built a theoretical compound in which the core, formed of long and thus weak carbon bonds, was stabilized by an outer shell of fused rings of the organic compound dibenzocycloheptatriene. Changing the structure of the side shell, for example by the bridging or non-bridging of the naphthalene skeleton, can lengthen the distance between the C-C bonds at the core.
The team then synthesized two compounds formed of colorless crystals, which they called 10a and 10b, and a third, called 10c, formed of orange crystals. X-ray analyses indicated long C-C bond lengths in all three compounds, with those in 10c reaching a record length of 1.806 angstroms when heated up to 127 °C (260.6 °F).
Although the longest bond was assumed to be unstable, the compound did not degrade even in a solution heated to high temperatures. This stability was supposedly given by the shells protecting the bond.
"By applying 'core-shell strategy' in molecular design, it is highly likely that we could find an even longer C-C bond," says Yusuke Ishigaki. "It's not just about breaking records, it's more about examining the fundamentals of chemistry."