New efficiency record set for perovskite LEDs
Researchers have set a new efficiency record for LEDs based on perovskite semiconductors, rivalling that of the best organic LEDs (OLEDs).

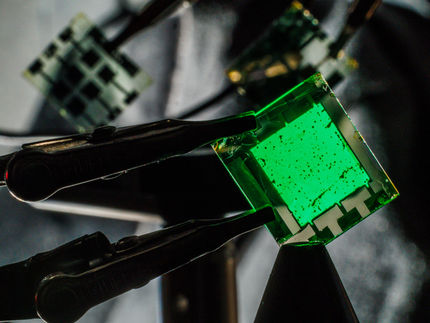
This is an artistic impression of the perovskite-polymer heterostructure used in LEDs.
University of Cambridge
Compared to OLEDs, which are widely used in high-end consumer electronics, the perovskite-based LEDs, developed by researchers at the University of Cambridge, can be made at much lower costs, and can be tuned to emit light across the visible and near-infrared spectra with high colour purity.
The researchers have engineered the perovskite layer in the LEDs to show close to 100% internal luminescence efficiency, opening up future applications in display, lighting and communications, as well as next-generation solar cells.
These perovskite materials are of the same type as those found to make highly efficient solar cells that could one day replace commercial silicon solar cells. While perovskite-based LEDs have already been developed, they have not been nearly as efficient as conventional OLEDs at converting electricity into light.
Earlier hybrid perovskite LEDs, first developed by Professor Sir Richard Friend's group at the University's Cavendish Laboratory four years ago, were promising, but losses from the perovskite layer, caused by tiny defects in the crystal structure, limited their light-emission efficiency.
Now, Cambridge researchers from the same group and their collaborators have shown that by forming a composite layer of the perovskites together with a polymer, it is possible to achieve much higher light-emission efficiencies, close to the theoretical efficiency limit of thin-film OLEDs.
"This perovskite-polymer structure effectively eliminates non-emissive losses, the first time this has been achieved in a perovskite-based device," said Dr Dawei Di from Cambridge's Cavendish Laboratory, one of the corresponding authors of the paper. "By blending the two, we can basically prevent the electrons and positive charges from recombining via the defects in the perovskite structure."
The perovskite-polymer blend used in the LED devices, known as a bulk heterostructure, is made of two-dimensional and three-dimensional perovskite components and an insulating polymer. When an ultra-fast laser is shone on the structures, pairs of electric charges that carry energy move from the 2D regions to the 3D regions in a trillionth of a second: much faster than earlier layered perovskite structures used in LEDs. Separated charges in the 3D regions then recombine and emit light extremely efficiently.
"Since the energy migration from 2D regions to 3D regions happens so quickly, and the charges in the 3D regions are isolated from the defects by the polymer, these mechanisms prevent the defects from getting involved, thereby preventing energy loss," said Di.
"The best external quantum efficiencies of these devices are higher than 20% at current densities relevant to display applications, setting a new record for perovskite LEDs, which is a similar efficiency value to the best OLEDs on the market today," said Baodan Zhao, the paper's first author.
While perovskite-based LEDs are beginning to rival OLEDs in terms of efficiency, they still need better stability if they are to be adopted in consumer electronics. When perovskite-based LEDs were first developed, they had a lifetime of just a few seconds. The LEDs developed in the current research have a half-life close to 50 hours, which is a huge improvement in just four years, but still nowhere near the lifetimes required for commercial applications, which will require an extensive industrial development programme. "Understand the degradation mechanisms of the LEDs is a key to future improvements," said Di.
Original publication
Baodan Zhao, Sai Bai, Vincent Kim, Robin Lamboll, Ravichandran Shivanna, Florian Auras, Johannes M. Richter, Le Yang, Linjie Dai, Mejd Alsari, Xiao-Jian She, Lusheng Liang, Jiangbin Zhang, Samuele Lilliu, Peng Gao, Henry J. Snaith, Jianpu Wang, Neil C. Greenham, Richard H. Friend & Dawei Di; "High-efficiency perovskite–polymer bulk heterostructure light-emitting diodes"; Nature Photonics; 2018