The Diffusion Interaction Parameter (kD) as an Indicator of Colloidal and Thermal Stability

Stability is a key quality attribute in formulation studies of po-tential therapeutic biomolecules. In order to minimize time, effort and funds spent on stability studies, researchers rely on high-throughput screening methods that can reliably test hundreds of combinations of candidates, excipients and buffer conditions. Techniques utilized in these screens must determine a variety of stability-indicating parameters (SIPs). Some of the most useful SIPs to date are short term aggre-gation, thermal stability; and colloidal stability.
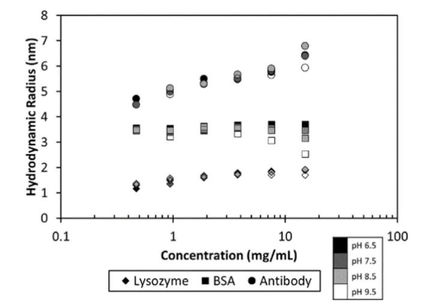
The ability to screen protein formulations at the early stages of development enables scientists to concentrate on the most suitable candidates, therefore saving substantial amounts of time, sample and testing equipment. This experiment demonstrates that thermal and colloidal stability of proteins, two indicators of propensity to aggregate, as well as actual aggregation states, are all determined simultaneously during the screening process with dynamic light scattering (DLS) tools in order to rank the effectiveness of candidates and for-mulation conditions. DLS can also indicate chemical stability and the average molar mass and specific volume of mole-cules in solution. For these reasons, HTS-DLS provides sub-stantial quantities of information for the rapid screening of candidate molecules, buffer conditions and excipients, allow-ing the DynaPro® Plate Reader III to maximize productivity in formulation studies.
Download white paper now
The Diffusion Interaction Parameter (kD) as an Indicator of Colloidal and Thermal Stability