To use all functions of this page, please activate cookies in your browser.
my.chemeurope.com
With an accout for my.chemeurope.com you can always see everything at a glance – and you can configure your own website and individual newsletter.
- My watch list
- My saved searches
- My saved topics
- My newsletter
Ammonium hydroxideAmmonium hydroxide, also known as ammonia water, aqua ammonia, or aqueous ammonia, is a solution of ammonia in water. Technically, the use of the term "ammonium hydroxide" is incorrect because such a chemical compound is not isolatable. However, this term does give a fair description of how an ammonia solution behaves, and is commonly used even by scientists and engineers. Product highlightAvailabilityAqueous ammonia is readily available from chemical supply houses in concentrations of about 30 %. Some household cleaning solutions may consist of dilute solutions of ammonia as well. Small chemistry kits also have trace amounts of this substance. ChemistryIn aqueous solution, ammonia deprotonates some small fraction of the water to give ammonium and hydroxide ions according to the following equilibrium:
With a base ionization constant (Kb) of 1.8×10-5, in a 1M ammonia solution about 0.42% of the ammonia will gain protons to become ammonium ions (equivalent to a pH of 11.63). Aqueous ammonia is used in traditional qualitative inorganic analysis. Like many amines, it gives a deep blue coloration with copper(II) solutions. Ammonia solution can dissolve silver residues, such as that formed from Tollens' reagent. Solutions of ammonium can also dissolve reactive metals such as aluminum and zinc, with the liberation of hydrogen gas. When ammonium hydroxide is mixed with dilute hydrogen peroxide in the presence of a metal ion, such as Cu2+, the peroxide will undergo rapid decomposition. See alsoCategories: Ammonium compounds | Bases |
| This article is licensed under the GNU Free Documentation License. It uses material from the Wikipedia article "Ammonium_hydroxide". A list of authors is available in Wikipedia. |





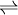 NH4+ + OH-
NH4+ + OH-


