To use all functions of this page, please activate cookies in your browser.
my.chemeurope.com
With an accout for my.chemeurope.com you can always see everything at a glance – and you can configure your own website and individual newsletter.
- My watch list
- My saved searches
- My saved topics
- My newsletter
Balmer's ConstantBalmer's Constant is used in chemistry to discern the frequency of light emitted when an atom's electron returns to the ground state. It can also be used to find the frequency of light necessary to excite an electron to a certain energy level. Balmer's Constant has a value of 3.29 * 1015 s-1 and is denoted with a capital C (not to be confused with c, the speed of light in a vacuum). It is used in the formula:
Product highlightν is the frequency of emitted/absorbed light, C is Balmer's constant, ni is the initial principal quantum number and nf is the final principle quantum number. The sign of the answer indicates whether the light is absorbed or emitted. A negative sign denotes emitted light where a positive sign denotes absorbed light.
See also
Categories: Hydrogen | Emission spectroscopy |
| This article is licensed under the GNU Free Documentation License. It uses material from the Wikipedia article "Balmer's_Constant". A list of authors is available in Wikipedia. |


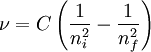 .
.





