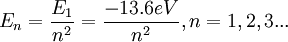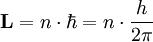To use all functions of this page, please activate cookies in your browser.
my.chemeurope.com
With an accout for my.chemeurope.com you can always see everything at a glance – and you can configure your own website and individual newsletter.
- My watch list
- My saved searches
- My saved topics
- My newsletter
Principal quantum numberIn atomic physics, the principal quantum number symbolized as n is the first of a set of quantum numbers (which includes: the principal quantum number, the azimuthal quantum number, the magnetic quantum number, and the spin quantum number) of an atomic orbital. The quantum number n labels the energy levels of hydrogenic atoms. It is the first in a set of numbers that show the unique quantum state of an electron. Labeling follows Spectroscopic notation Product highlightDerivationThere are a set of quantum numbers associated with the energy states of the atom. The four quantum numbers n, l, m, and s specify the complete and unique quantum state of a single electron in an atom called its wavefunction or orbital. No two electrons belonging to the same atom can have the same four quantum numbers which is shown in the Pauli Exclusion Principle. The wavefunction of the Schrödinger wave equation reduces to the three equations that when solved lead to the first three quantum numbers. Therefore, the equations for the first three quantum numbers are all interrelated. The principal quantum number arose in the solution of the radial part of the wave equation as shown below. The Schrödinger wave equation describes energy eigenstates having corresponding real numbers En with a definite total energy which the value of En defines. The bound state energies of the electron in the hydrogen atom are given by: The parameter n can take only positive integer values. This idea of energy levels and notation was borrowed from the earlier Bohr model of the atom and expanded in Schrödinger's equation from the flat two-dimensional Bohr atom to the three-dimensional wavefunction model of the atom. The allowed orbits depend on quantized (discrete) values of orbital angular momentum, L according to the equation
The energy of any wave is the frequency multiplied by Planck's constant. This causes the wave to display particle-like packets of energy called quanta. To show each of the quantum numbers in the quantum state, the formulae for each quantum number include Planck's reduced constant which only allows particular or discrete or quantized energy levels. The principal quantum number n represents the relative overall energy of each orbital and the energy of each orbital increases as the distance from the nucleus increases. The sets of orbitals with the same n-value are often referred to as electron shells or energy levels. In the notation of the periodic table, the main shells of electrons are labeled: K(n=1), L(n=2), M(n=3), etc. based on the principal quantum number. The principle quantum number is related to the radial quantum number, nr, by: n = nr + l + 1 where l is the azimuthal quantum number and nr is equal to the number of nodes in the radial wavefunction. See also
External references
Categories: Quantum chemistry | Atomic physics |
| This article is licensed under the GNU Free Documentation License. It uses material from the Wikipedia article "Principal_quantum_number". A list of authors is available in Wikipedia. |