To use all functions of this page, please activate cookies in your browser.
my.chemeurope.com
With an accout for my.chemeurope.com you can always see everything at a glance – and you can configure your own website and individual newsletter.
- My watch list
- My saved searches
- My saved topics
- My newsletter
Blood sugarBlood sugar is a term used to refer to the amount of glucose in the blood. Glucose, transported via the bloodstream, is the primary source of energy for the body's cells. Blood sugar concentration, or glucose level, is tightly regulated in the human body. Normally, the blood glucose level is maintained between about 4 and 8 mmol/L (70 to 150 mg/dL). The total amount of glucose in the circulating blood is therefore about 3.3 to 7g (assuming an ordinary adult blood volume of 5 liters). Glucose levels rise after meals and are usually lowest in the morning, before the first meal of the day. Failure to maintain blood glucose in the normal range leads to conditions of persistently high (hyperglycemia) or low (hypoglycemia) blood sugar. Diabetes mellitus, characterized by persistent hyperglycemia of several causes, is the most prominent disease related to failure of blood sugar regulation. Although it is called "blood sugar," sugars besides glucose are found in the blood, such as fructose and galactose. Only glucose levels are regulated via insulin and glucagon. Product highlight
Glucose measurementSample typeGlucose can be measured in whole blood, serum, or plasma. Historically, blood glucose values were given in terms of whole blood, but most laboratories now measure and report the serum glucose levels. Because RBC (erythrocytes) have a higher concentration of protein (i.e. hemoglobin) than serum, serum has a higher water content and consequently more dissolved glucose than does whole blood. To convert from whole-blood glucose, multiply the value by 1.15 to give the serum/plasma level. Collection of blood in clot (red-top) tubes for serum chemistry analysis permits the metabolism of glucose in the sample by blood cells until separated by centrifugation. Higher than normal amounts of white or red blood cell counts can lead to excessive glycolysis in the sample with substantial reduction of glucose level if the sample is not processed quickly. Ambient temperature at which the blood sample is kept prior to centrifugation and separation of Plasma/Serum also affects glucose levels. At refrigerator temperatures, glucose remains relatively stable for several hours in the blood sample. At room temperature (25°C), a loss of 1 to 2% of glucose per hour should be expected. The loss of glucose levels in aforementioned conditions can be prevented by using Fluoride top (gray-top) as the anticoagulant of choice upon blood collection, as Fluoride inhibits glycolysis. However, this should only be used when blood will be transported from one hospital laboratory to another for glucose measurement. Red-top serum separator tubes also preserve glucose in samples once they have been centrifugated to isolate the serum from cells, this tube would be the most efficient. Particular care should be given to drawing blood samples from the arm opposite the one in which an intravenous line is inserted, to prevent contamination of the sample with intravenous fluids (IV). Alternatively, blood can be drawn from the same arm with an IV line after the IV was turned off for at least 5 minutes and the arm is elevated to drain the infused fluids away from the vein. As little as 10% contamination with 5% dextrose (D5W) will elevate glucose in a sample by 500mg/dl or more. Arterial, capillary and venous blood have comparable glucose levels in a fasting individual, whereas after meals venous levels are lower than capillary or arterial blood. MethodologyThere are two different major methods that have been used to measure glucose. The older one is a chemical method that exploits the nonspecific reducing property of glucose in a reaction with an indicator substance that acquires or changes color on its reduction. Since other blood compounds also have reducing properties (e.g., urea, which can build up in uremic patients), this method can have erroneous measurements up to 5 to 15 mg/dl. This is solved by the Enzymatic methods that are highly specific for glucose. The two most common employed enzymes are glucose oxidase and hexokinase.
Laboratory tests
Clinical correlationThe fasting blood glucose (FBG) level is the most commonly used indication of overall glucose homeostasis. Conditions that affect glucose levels are shown in the table below. They reflect abnormalities in the multiple control mechanism of glucose regulation. The metabolic response to a carbohydrate challenge is conveniently assessed by the postprandial glucose level drawn 2 hours after a meal or a glucose load. In addition, the glucose tolerance test, consisting of serial timed measurements after a standardized amount of oral glucose intake, is used to aid in the diagnosis of Diabetes.
Health effectsIf blood sugar levels drop too low, a potentially fatal condition called hypoglycemia develops. Symptoms may include lethargy, impaired mental functioning, irritability, and loss of consciousness. If levels remain too high, appetite is suppressed over the short term. Long-term hyperglycemia causes many of the long-term health problems associated with diabetes, including eye, kidney, and nerve damage. Low blood sugarSome people report drowsiness or impaired cognitive function several hours after meals, which they believe is related to a drop in blood sugar, or "low blood sugar". For more information, see:
Converting glucose unitsCountries that use the metric system are gradually changing to mmol/L. The U.S. uses mg/dL. To convert blood glucose readings:
References
Categories: Blood tests | Diabetes |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| This article is licensed under the GNU Free Documentation License. It uses material from the Wikipedia article "Blood_sugar". A list of authors is available in Wikipedia. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||





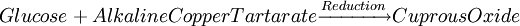
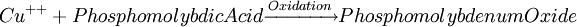
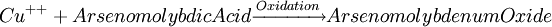
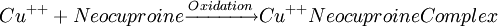 *
*
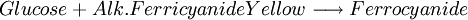
![Glucose + O^{2}\xrightarrow[Oxidation] {glucose oxidase}Cuprous Oxide](images/math/4/c/c/4cc01f225894a222df624e7a91bb3c9a.png)
![H_{2}O_2 + O-dianisidine\xrightarrow[Oxidation] {peroxidase} H_2O + oxidized chromogen](images/math/2/7/2/27206745872a5c8034d50090c7139632.png)
![\begin{alignat}{2} & Glucose + ATP\xrightarrow[Phosphorylation] {Hexokinase + Mg^{++}} G-6PO_4 + ADP \\ & G-6PO_4 + NADP\xrightarrow[Oxidation] {G-6PD} G-Phosphogluconate + NADPH + H^{+} \\ \end{alignat}](images/math/7/9/4/79469869930e089fce4ccbca1db3fdfe.png)


