To use all functions of this page, please activate cookies in your browser.
my.chemeurope.com
With an accout for my.chemeurope.com you can always see everything at a glance – and you can configure your own website and individual newsletter.
- My watch list
- My saved searches
- My saved topics
- My newsletter
Boudouard reactionBoudouard reaction is the redox reaction of chemical equilibrium mixture of carbon monoxide and carbon dioxide in a given temperature. It is the disproportionation of carbon monoxide into carbon dioxide and graphite or its reverse
Product highlightAccording to the Ellingham diagram, the formation enthalpy of CO2 by oxidation of carbon is constant and indifferent of the temperature, while the formation enthalpy of CO is a decreasing line. The Boudouard reaction implies that on lower temperatures the equilibrium is on the exothermic carbon dioxide side and on higher temperatures the endothermic formation of carbon monoxide is the dominant product, as predicted by the Le Chatelier's Principle. For instance, in the high-temperature, reducing environment of a smokestack, carbon monoxide is the stable product. When the carbon monoxide reaches the top of the smokestack, and the cooler air, the Boudouard Reaction takes place, the carbon monoxide is oxidized into carbon dioxide, and the graphite precipitates (reduces) as soot. The Ellingham diagram defines the equilibrium formation enthalpy on function of temperature. In industrial catalysis, this is not just an eyesore; the coking can cause irreversible damage to catalysts and catalyst beds. This reaction takes also place in blast furnace where carbon monoxide is used as the reductive agent on purifying metallic iron from its oxides in ore. |
| This article is licensed under the GNU Free Documentation License. It uses material from the Wikipedia article "Boudouard_reaction". A list of authors is available in Wikipedia. |


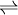 CO2 + C
CO2 + C





