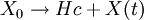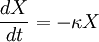To use all functions of this page, please activate cookies in your browser.
my.chemeurope.com
With an accout for my.chemeurope.com you can always see everything at a glance – and you can configure your own website and individual newsletter.
- My watch list
- My saved searches
- My saved topics
- My newsletter
Catagenesis (geology)See Catagenesis (biology) for usage in the field of biology, where it refers to retrogressive evolution. Contrast with anagenesis.
Catagenesis is a term used in petroleum geology to describe the cracking process which results in the conversion of organic kerogens into hydrocarbons. Theoretical reactionThis chemical reaction is believed to be a time, temperature and pressure dependent process which creates liquid and/or gaseous hydrocarbon Hc from primary kerogen X and can be summarised using the formula: where X0 is the initial kerogen concentration and X(t) is the kerogen concentration at time t. It is generally held that the dependence on pressure is negligible, such that the process of catagenesis can be given as a first-order differential equation: where X is the reactant (kerogen) and κ is the reaction-rate constant which introduces the temperature-dependence via the Arrhenius equation. Important parametersSeveral generally unrecognized but important controlling parameters of metamorphism have been suggested.[1]
Erroneous assumptionA large body of petroleum-geochemical data suggests these are not first-order reactions.[1] This means that geologic time has a minimal role.
See also
References |
||
| This article is licensed under the GNU Free Documentation License. It uses material from the Wikipedia article "Catagenesis_(geology)". A list of authors is available in Wikipedia. |