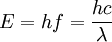To use all functions of this page, please activate cookies in your browser.
my.chemeurope.com
With an accout for my.chemeurope.com you can always see everything at a glance – and you can configure your own website and individual newsletter.
- My watch list
- My saved searches
- My saved topics
- My newsletter
Colors of chemicals
All chemical compounds have colors, and in some cases these are distinctive or useful for identification. The study of chemical structure by means of energy adsorption and release is generally referred to as spectroscopy. Product highlight
TheoryAll chemical compounds, including atoms, are capable of absorbing and releasing energy. The amount(s) of energy (quanta) absorbed and released is determined by the quantum structure of the chemical. The release of energy visible to the human eye spans the wavelengths 380 nm to 760 nm and is commonly referred to as color. The relationship between energy and wavelength is determined by the equation: where E is the energy of the quanta (photon), h is Planck's constant, λ is the wavelength and c is the speed of light. The relationship between chemical structure and energy can be understood using atomic orbital, molecular orbital, or Ligand Field Theory. In organic compounds the color can be determined by the difference between the Highest Occupied Molecular Orbital and the Lowest Unoccupied Molecular Orbital. The energy absorbed and/or released does not directly correlate to what humans perceive as color. The absorption of a particular wavelength of light effectively subtracts it from the full visible spectrum, and what we see is the complementary color, made up of the other visible wavelengths. Beta-carotene has maximum absorption at 454 nm (blue light) consequently what visible light remains appears orange. Colors by wavelengthBelow is a rough table of wavelengths, colors and complementary colors.
ExamplesIons in aqueous solution
It is important to note, however, that elemental colors will vary depending on what they are complexed with, often as well as their chemical state. An example with vanadium(III); VCl3 has a distinctive redish hue, whilst V2O3 appears black. SaltsPredicting the color of a compound can be extremely complicated. Some examples include: Cobalt chloride is pink or blue depending on the state of hydration (blue dry, pink with water) so it's used as a moisture indicator in silica gel. Zinc Oxide is white, but at higher temperatures becomes yellow, returning to white as it cools.
Oxidising Metals
Oxidising Gases
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| This article is licensed under the GNU Free Documentation License. It uses material from the Wikipedia article "Colors_of_chemicals". A list of authors is available in Wikipedia. |