To use all functions of this page, please activate cookies in your browser.
my.chemeurope.com
With an accout for my.chemeurope.com you can always see everything at a glance – and you can configure your own website and individual newsletter.
- My watch list
- My saved searches
- My saved topics
- My newsletter
Compressibility factorThe compressibility factor (Z) is used to alter the ideal gas equation to account for the real gas behaviour.[1] The compressibility factor is usually obtained from the compressibility chart.[2] Product highlightMathematically, it is defined as, where, P is the pressure,
For ideal gas behaviour, Z = 1. Ideal gas behaviour occurs when,
where,
The deviation from ideal gas behaviour is largest in the vicinity of the critical point, where Z can be as low as 0.2. See alsoReferences
Categories: Chemical engineering | Thermodynamics |
| This article is licensed under the GNU Free Documentation License. It uses material from the Wikipedia article "Compressibility_factor". A list of authors is available in Wikipedia. |





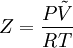
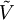 is the
is the 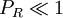
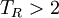 (except when
(except when 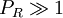 )
)
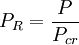 is the
is the 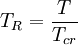 is the
is the 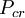 and
and 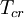 are the
are the 

