To use all functions of this page, please activate cookies in your browser.
my.chemeurope.com
With an accout for my.chemeurope.com you can always see everything at a glance – and you can configure your own website and individual newsletter.
- My watch list
- My saved searches
- My saved topics
- My newsletter
DNA meltingDNA melting, also called DNA denaturation, is the process by which double-stranded deoxyribonucleic acid unwinds and separates into single-stranded strands through the breaking of hydrogen bonding between the bases. Both terms are used to refer to the process as it occurs when a mixture is heated, although "denaturation" can also refer to the separation of DNA strands induced by chemicals like urea. For multiple copies of DNA molecules, the melting temperature (Tm) is defined as the temperature at which half of the DNA strands are in the double-helical state and half are in the "random-coil" states.[1] The melting temperature depends on both the length of the molecule, and the specific nucleotide sequence composition of that molecule. Product highlight
Applications of DNA denaturationThe process of DNA denaturation can be used to analyze some aspects of DNA. Because cytosine / guanine base-pairing is generally stronger than adenosine / thymine base-pairing, the amount of cytosine and guanine in a genome (called the "GC content") can be estimated by measuring the temperature at which the genomic DNA melts.[2] Higher temperatures are associated with high GC content. DNA denaturation can also be used to detect sequence differences between two different DNA sequences. DNA is heated and denatured into single-stranded state, and the mixture is cooled to allow strands to rehybridize. Hybrid molecules are formed between similar sequences and any differences between those sequences will result in a disruption of the base-pairing. On a genomic scale, the method has been used by researchers to estimate the genetic distance between two species, a process known as DNA-DNA hybridization.[3] In the context of a single isolated region of DNA, denaturing gradient gels and temperature gradient gels can be used to detect the presence of small mismatches between two sequences, a process known as temperature gradient gel electrophoresis.[4][5] Methods of DNA analysis based on melting temperature have the disadvantage of being proxies for studying the underlying sequence; DNA sequencing is generally considered a more accurate method. The process of DNA melting is also used in molecular biology techniques, notably in the polymerase chain reaction (PCR). Although the temperature of DNA melting is not diagnostic in the technique, methods for estimating Tm are important for determining the appropriate temperatures to use in a protocol. DNA melting temperatures can also be used as a proxy for equalizing the hybridization strengths of a set of molecules, eg. the oligonucleotide probes of DNA microarrays. Methods for estimating TmSeveral formulas are used to calculate Tm values.[6][7] Some formulas are more accurate in predicting melting temperatures of DNA duplexes.[8] Wallace methodThe fastest and less accurate Wallace method is suitable for oligos less than 18mers in length by counting the frequency of each nucleotide base. The reasoning behind the method is that, because cytosine-guanine pairs form three hydrogen bonds compared to the two hydrogen bonds between adenosine and thymine, they contribute more to the stability of a double-helix.
Nearest-neighbor methodThis is far more accurate method used to predict melting temperatures of nucleic acid duplexes. Although GC content plays a large factor in the hybridization energy of double-stranded DNA, interactions between neighboring bases along the helix means that stacking energies are significant. The nearest-neighbor model accounts for this by considering adjacent bases along the backbone two-at-a-time.[1] Each of these has enthalpic and entropic parameters, the sums of which determine melting temperature according to the following equation:
Standard enthalpies and entropies are negative for the annealing reaction and are assumed to be temperature independent. If C1 > > C2 then C2 can be neglected.
References
See also
Categories: DNA | Nucleic acids |
||||||||||||||||||||||||||||||||||||||||
| This article is licensed under the GNU Free Documentation License. It uses material from the Wikipedia article "DNA_melting". A list of authors is available in Wikipedia. |





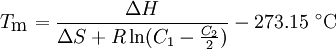
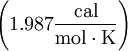 .
.


