To use all functions of this page, please activate cookies in your browser.
my.chemeurope.com
With an accout for my.chemeurope.com you can always see everything at a glance – and you can configure your own website and individual newsletter.
- My watch list
- My saved searches
- My saved topics
- My newsletter
ElastomerAn elastomer is a polymer with the property of elasticity. The term, which is derived from elastic polymer, is often used interchangeably with the term rubber, and is preferred when referring to vulcanisates. Each of the monomers which link to form the polymer is usually made of carbon, hydrogen, oxygen and/or silicon. Elastomers are amorphous polymers existing above their glass transition temperature, so that considerable segmental motion is possible. At ambient temperatures rubbers are thus relatively soft (E~3MPa) and deformable. Their primary uses are for seals, adhesives and molded flexible parts. Product highlight
BackgroundElastomers are usually thermosets (requiring vulcanization) but may also be thermoplastic (see thermoplastic elastomer). The long polymer chains cross-link during curing. The molecular structure of elastomers can be imagined as a 'spaghetti and meatball' structure, with the meatballs signifying cross-links. The elasticity is derived from the ability of the long chains to reconfigure themselves to distribute an applied stress. The covalent cross-linkages ensure that the elastomer will return to its original configuration when the stress is removed. As a result of this extreme flexibility, elastomers can reversibly extend from 5-700%, depending on the specific material. Without the cross-linkages or with short, uneasily reconfigured chains, the applied stress would result in a permanent deformation. Temperature effects are also present in the demonstrated elasticity of a polymer. Elastomers that have cooled to a glassy or crystalline phase will have less mobile chains, and consequentially less elasticity, than those manipulated at temperatures higher than the glass transition temperature of the polymer. It is also possible for a polymer to exhibit elasticity that is not due to covalent cross-links, but instead for thermodynamic reasons. Mathematic justificationsUsing the laws of thermodynamics, stress definitions and polymer characteristics (complete derivation in [1], pages103-105), we find ideal stress behavior:
where n is the number of chain segments per unit volume, k is Boltzmann's Constant, T is temperature, and These findings are accurate for values of up to approximately 400% strain. At this point, alignment between stretched chains begins to result in crystallization from noncovalent bonding. While Young's Modulus does not exist for elastomers due to the nonlinear nature of the stress-strain relationship, a "secant modulus" can be found at a particular strain. Examples of elastomersUnsaturated rubbers that can be cured by sulfur vulcanization:
(Note that unsaturated rubbers can also be cured by non-sulfur vulcanization if desired). Saturated Rubbers that cannot be cured by sulfur vulcanization:
Various other types of elastomers:
References
Categories: Elastomers | Materials science | Polymers |
|
| This article is licensed under the GNU Free Documentation License. It uses material from the Wikipedia article "Elastomer". A list of authors is available in Wikipedia. |





![\sigma\ = n k T [ \lambda\ _ 1 ^ 2 + \lambda\ _ 1 ^ {-1} ]](images/math/2/a/f/2af12e1879f096d2e92fa53e4aac6856.png)
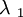 is distortion in the 1 direction.
is distortion in the 1 direction.


