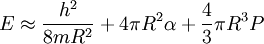To use all functions of this page, please activate cookies in your browser.
my.chemeurope.com
With an accout for my.chemeurope.com you can always see everything at a glance – and you can configure your own website and individual newsletter.
- My watch list
- My saved searches
- My saved topics
- My newsletter
Electron bubbleAn electron bubble is the empty space created around a free electron in a cryogenic gas or liquid, such as neon or helium. They are typically very small, about 2 nm in diameter at atmospheric pressure. Product highlight
Electron bubbles in heliumAt room temperature, electrons in noble gasses move about freely, limited only by collisions with the weakly interacting atoms. Their mobility, which depends on the gas density and temperature, is well described by classical kinetic theory. As the temperature is lowered the electron mobility increases, since the helium atoms slow down at lower temperature and do not interact with the electron as often[1]. Below a critical temperature, the mobility of the electrons drops quickly to a value much below what is expected classically. This discrepancy led to the development of the electron bubble theory[2]. At low temperatures, electrons injected into liquid helium do not move freely as one might expect, but rather form small vacuum bubbles around themselves. Electron repulsion from the surface of heliumElectrons are attracted to liquid helium due to the difference in dielectric constants between the gas and liquid phase of helium. The negative electron polarizes the helium at the surface, leading to an image charge which binds it to the surface. The electron is forbidden from entering the liquid for the same reason hydrogen atoms are stable: quantum mechanics. The electron and image charge form a bound state, just as an electron and proton do in a hydrogen atom, with a minimum average separation. In this case, the minimum energy is about 1eV (a moderate amount of energy on an atomic scale)[3]. When an electron is forced into liquid helium rather than floating on its surface, it forms a bubble rather than enter the liquid. The size of this bubble is determined by three main factors (ignoring small corrections): the confinement term, the surface tension term, and the pressure-volume term. The confinement term is purely quantum mechanical, since whenever an electron is tightly confined, its kinetic energy goes up. The surface tension term represents the surface energy of the liquid helium; this is exactly like water and all other liquids. The pressure-volume term is the amount of energy needed to push the helium out of the bubble[4].
Here E is the energy of the bubble, h is Planck's constant, m is the electron mass, R is the bubble radius, α is the surface energy, and P is the ambient pressure. Splitting the electron bubbleWhen an electron becomes excited, its wave function in the 1P state becomes "dumb-bell" shaped and therefore so too does its electron bubble. British physicist Humphrey Maris had the idea "if the dumb-bell could be stretched and pinched, might it simply divide?" Maris calculated that light with a wavelength of around 10 micrometres would excite the electron into its "dumb-bell" shaped bubble which, under great pressure, would split at its thinnest point giving two "half bubbles" with half an electron in each. However, in modern quantum mechanics there is no such thing as a "half-electron," leading to much scepticism from experts in quantum theory although none of them have been able to disprove Maris's theory. It seems either Maris or quantum theory must be wrong as the theories are mutually incompatible. The 2S electron bubbleA theoretical prediction has been made based on the analysis of the equation above [5], that the 2S electron bubble exhibits a startling morphological instability under a wide range of ambient pressures. While its wave function is spherical, the stable shape of the bubble is nonspherical.
|
|
| This article is licensed under the GNU Free Documentation License. It uses material from the Wikipedia article "Electron_bubble". A list of authors is available in Wikipedia. |