To use all functions of this page, please activate cookies in your browser.
my.chemeurope.com
With an accout for my.chemeurope.com you can always see everything at a glance – and you can configure your own website and individual newsletter.
- My watch list
- My saved searches
- My saved topics
- My newsletter
Forward osmosisForward Osmosis is an osmotic process that, like reverse osmosis, uses a semi-permeable membrane to effect separation of water from dissolved solutes. The driving force for this separation is an osmotic pressure gradient, such that a "draw" solution of high concentration (relative to that of the feed solution), is used to induce a net flow of water through the membrane into the draw solution, thus effectively separating the feed water from its solutes. In contrast, the reverse osmosis process uses hydraulic pressure as the driving force for separation, which serves to counteract the osmotic pressure gradient that would otherwise favor water flux from the permeate to the feed. The simplest equation describing the relationship between osmotic and hydraulic pressures and water flux is: 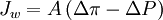 Product highlightwhere Jw is water flux, A is the hydraulic permeability of the membrane, Δπ is the difference in osmotic pressures on the two sides of the membrane, and ΔP is the difference in hydrostatic pressure (negative values of Jw indicating reverse osmotic flow). The modeling of these relationships is in practice more complex than this equation indicates, with flux depending on the membrane, feed, and draw solution characteristics, as well as the fluid dynamics within the process itself [1]. An additional distinction between the reverse osmosis (RO) and forward osmosis (FO) processes is that the water permeating the RO process is in most cases fresh water ready for use. In the FO process, this is not the case. The membrane separation of the FO process in effect results in a "trade" between the solutes of the feed solution and the draw solution. Depending on the concentration of solutes in the feed (which dictates the necessary concentration of solutes in the draw) and the intended use of the product of the FO process, this step may be all that is required. One example of an application of this type may be found in "hydration bags", which use an ingestible draw solute and are intended for separation of water from dilute feeds. This allows, for example, the ingestion of water from surface waters (streams, ponds, puddles, etc) that may be expected to contain pathogens or toxins that are readily rejected by the FO membrane. With sufficient contact time, such water will permeate the membrane bag into the draw solution, leaving the undesirable feed constituents behind. The diluted draw solution may then be ingested directly. Typically, the draw solutes are sugars such as glucose or fructose, which provide the additional benefit of nutrition to the user of the FO device. A point of additional interest with such bags is that they may be readily used to recycle urine, greatly extending the ability of a backpacker or soldier to survive in arid environments [2]. This process may also, in principle, be employed with highly concentrated saline feedwater sources such as seawater, as one of the first intended uses of FO with ingestible solutes was for survival in liferafts at sea [3]. In the case where fresh water which does not contain draw solutes is the desired product, a second separation step is required. The first separation step of FO, driven by an osmotic pressure gradient, does not require a significant energy input (only unpressurized stirring or pumping of the solutions involved). The second separation step, however does typicially require energy input. One method used for the second separation step is to employ RO. This approach has been used, for instance, in the treatment of landfill leachate. A FO membrane separation is used to draw water from the leachate feed into a saline (NaCl) brine. The diluted brine is then passed through a RO process to produce fresh water and a reusable brine concentrate. The advantage of this method is not a savings in energy, but rather in the fact that the FO process is more resistant to fouling from the leachate feed than a RO process alone would be [4] (see: Osmotek). A similar FO / RO hybrid has been used for the concentration of food products, such as fruit juice [5]. One area of current research in FO involves the direct removal of draw solutes by thermal means. This process is typically referred to as the "ammonia - carbon dioxide" FO process, as the draw solutes are salts formed from the mixing of ammonia and carbon dioxide gases in water [6]. These salts can reach high concentrations, particularly as the ratio of ammonia to carbon dioxide is increased. An especially convenient property of these salts is that they readily dissociate into ammonia and carbon dioxide gases again, if a solution containing them is heated (to approx. 60°C, at 1 atm pressure). Once the concentrated draw solution is used to effect separation of water from the FO feed solution, the diluted draw solution is directed to a reboiled stripper (distillation column) and the solutes are completely removed and recycled for reuse in the FO system [7]. An FO system of this type thereby effects membrane separation of water from the FO feed, using heat as its primary energy source. The quality of heat used by this process can be very low, at temperatures as low as 40°C. If FO of this type is used in a cogeneration environment (waste heat from a power plant, for example), its energy cost can be greatly reduced compared to RO [8]. (See: Yale University .) A second area of current research in FO also involves direct removal of draw solutes, in this case by means of a magnetic field. Small (nano scale) magnetic particles are suspended in solution creating osmotic pressures sufficient for the separation of water from a dilute feed. Once the draw solution containing these particles has been diluted by the FO water flux, they may be separated from that solution by use of a magnet (either against the side of a hydration bag, or around a pipe in-line in a steady state process). (See: Apaclara.) A comprehensive review of publications discussing forward osmosis has recently been published, by researchers at the University of Nevada, Reno, and Yale: Cath et al. [9]. References[1] K.L. Lee, R.W. Baker, H.K. Lonsdale, Membranes for power-generation by pressure-retarded osmosis, J. Membr. Sci. 8 (1981) 141–171. [2] Salter, R.J., Forward Osmosis, Water Conditioning and Purification 48 (4) (2005) 36-38. [3] J. O. Kessler and C. D. Moody, Drinking water from sea water by forward osmosis, Desalination, 18 (1976) 297-306. [4] R. J. York, R. S. Thiel and E. G. Beaudry, Full-scale experience of direct osmosis concentration applied to leachate management, Sardinia ’99 Seventh International Waste Management and Landfill Symposium, S. Margherita di Pula, Cagliari, Sardinia, Italy, 1999. [5] E. G. Beaudry and K. A. Lampi, Membrane technology for direct osmosis concentration of fruit juices, Food Technology, 44 (1990) 121. [6] J.R. McCutcheon, R.L. McGinnis, M. Elimelech, A novel ammonia - carbon dioxide forward (direct) osmosis desalination process, Desalination 174 (2005) 1–11. [7] J. R. McCutcheon, R. L. McGinnis and M. Elimelech, Desalination by a novel ammonia-carbon dioxide forward osmosis process: Influence of draw and feed solution concentrations on process performance, J. Membr. Sci. 278 (2006) 114-123. [8] R.L. McGinnis, Energy Requirements of Ammonia–Carbon Dioxide Forward Osmosis Desalination, Poster Presentation, North American Membrane Society (2006). [9] T. Y. Cath, A. E. Childress and M. Elimelech, Forward osmosis: Principles, applications, and recent developments, J. Membr. Sci. 281 (2006) 70-87. Categories: Diffusion | Water technology |
| This article is licensed under the GNU Free Documentation License. It uses material from the Wikipedia article "Forward_osmosis". A list of authors is available in Wikipedia. |







