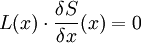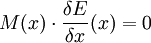To use all functions of this page, please activate cookies in your browser.
my.chemeurope.com
With an accout for my.chemeurope.com you can always see everything at a glance – and you can configure your own website and individual newsletter.
- My watch list
- My saved searches
- My saved topics
- My newsletter
GENERIC formalismIn non-equilibrium thermodynamics, GENERIC is an acronym for General Equation for Non-Equilibrium Reversible-Irreversible Coupling. It is the general form of dynamic equation for a system with both reversible and irreversible dynamics (generated by energy and entropy, respectively). GENERIC formalism is the theory built around the GENERIC equation, which has been proposed in its final form in 1997 by Miroslav Grmela and Hans Christian Öttinger. Product highlightGENERIC equationThe GENERIC equation is usually written as Here:
In addition to the above equation and the properties of its constituents, systems that ought to be properly described by the GENERIC formalism are required to fulfill the degeneracy conditions which express the conservation of entropy under reversible dynamics and of energy under irreversible dynamics, respectively. The conditions on L (antisymmetry and some others) express that the energy is reversibly conserved, and the condition on M (positive semidefiniteness) express that the entropy is irreversibly non-decreasing. References
|
| This article is licensed under the GNU Free Documentation License. It uses material from the Wikipedia article "GENERIC_formalism". A list of authors is available in Wikipedia. |





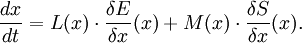
 , where the set
, where the set 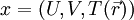 for a gas with nonuniform temperature, contained in a volume
for a gas with nonuniform temperature, contained in a volume 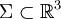 (
(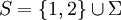 )
)
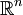 to
to 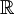 , for continuously indexed
, for continuously indexed