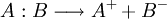To use all functions of this page, please activate cookies in your browser.
my.chemeurope.com
With an accout for my.chemeurope.com you can always see everything at a glance – and you can configure your own website and individual newsletter.
- My watch list
- My saved searches
- My saved topics
- My newsletter
HeterolysisIn chemistry, heterolysis or heterolytic fission (from Greek ἑτερος, heteros, "different," and λυσις, lusis, "loosening") is chemical bond cleavage of a neutral molecule generating a cation and an anion.[1] In this process the two electrons that make up the bond are assigned to the same fragment. Product highlightThe energy involved in this process is called heterolytic bond dissociation energy. Bond cleavage is also possible by a process called homolysis. In heterolysis additional energy is required to separate the ion pair. An ionising solvent helps reduce this energy. In biology, heterolysis refers to necrosis induced by hydrolytic enzymes from surrounding (usually inflammatory) cells. Autolysis is necrosis of a cell by its own enzymes. See also
References
|
| This article is licensed under the GNU Free Documentation License. It uses material from the Wikipedia article "Heterolysis". A list of authors is available in Wikipedia. |