To use all functions of this page, please activate cookies in your browser.
my.chemeurope.com
With an accout for my.chemeurope.com you can always see everything at a glance – and you can configure your own website and individual newsletter.
- My watch list
- My saved searches
- My saved topics
- My newsletter
Hyperfine structureIn atomic physics, hyperfine structure is a small perturbation in the energy levels (or spectra) of atoms or molecules due to the magnetic dipole-dipole interaction, arising from the interaction of the nuclear magnetic moment with the magnetic field of the electron. Product highlight
TheoryAccording to classical thinking, the electron moving around the nucleus has a magnetic dipole moment, because it is charged. The interaction of this magnetic dipole moment with the magnetic moment of the nucleus (due to its spin) leads to hyperfine splitting. However, due to the electron's spin, there is also hyperfine splitting for s-shell electrons, which have zero orbital angular momentum. In this case, the magnetic dipole interaction is even stronger, as the electron probability density does not vanish inside the nucleus ( The amount of correction to the Bohr energy levels due to hyperfine splitting of the hydrogen atom is of the order of where
For atoms other than hydrogen, the nuclear spin where
This interaction obeys the Lande interval rule: The energy level is split into Usually, In a more advanced treatment, one also has to take the nuclear magnetic quadrupole moment into account. This is sometimes (?) referred to as "hyperfine structure anomaly". HistoryThe optical hyperfine structure was already observed in 1881 by Albert Abraham Michelson. It could, however, only be explained in terms of quantum mechanics in the 1920s. Wolfgang Pauli proposed the existence of a small nuclear magnetic moment in 1924. In 1935, M. Schiiler and T. Schmidt proposed the existence of a nuclear quadrupole moment in order to explain anomalies in the hyperfine structure. ApplicationsAstrophysicsAs the hyperfine splitting is very small, the transition frequencies usually are not optical, but in the range of radio- or microwave frequencies. Hyperfine structure gives the 21 cm line observed in HI region in interstellar medium. Carl Sagan and Frank Drake considered the hyperfine transition of hydrogen to be a sufficiently universal phenomenon so as to be used as a base unit of time and length on the Pioneer plaque and later Voyager Golden Record. In radio astronomy, heterodyne receivers are widely used in detection of the electromagnetic signals from celestial objects. The separations among various components of a hyperfine structure are usually small enough to fit into the receiver's IF band. Because optical depth varies with frequency, strength ratios among the hyperfine components differ from that of their intrinsic intensities. From this we can derive the object's physical parameters.[1] Nuclear technologyThe AVLIS process uses the hyperfine splitting of between optical transitions in uranium-235 and uranium-238 to selectively photoionize only the uranium-235 atoms and then separate the ionized particles from the non-ionized ones. Precisely tuned dye lasers are used as the sources of the necessary exact wavelength radiation. Use in defining the SI second and meterThe hyperfine structure transition can be used to make a microwave notch filter with very high stability, repeatability and Q factor, which can thus be used as a basis for very precise atomic clocks. Typically, the hyperfine structure transition frequency of a particular isotope of caesium or rubidium atoms is used as a basis for these clocks. Due to the accuracy of hyperfine structure transition-based atomic clocks, they are now used as the basis for the definition of the second. One second is now defined to be exactly 9,192,631,770 cycles of the hyperfine structure transition frequency of caesium-133 atoms. Since 1983, the meter is defined by declaring the speed of light in a vacuum to be exactly 299,792,458 metres per second. Thus: The metre is the length of the path travelled by light in vacuum during a time interval of 1/299 792 458 of a second. Precision tests of quantum electrodynamicsThe hyperfine splitting in hydrogen and in muonium have been used to measure the value of the fine structure constant α. Comparison with measurements of α in other physical systems provides a stringent test of QED. Qubit in ion-trap quantum computingThe hyperfine states of a trapped ion are commonly used for storing qubits in ion-trap quantum computing. They have the advantage of having a very long lifetimes, experimentally exceeding ~10 min (compared to ~1 s for metastable electronic levels). The frequency associated with the states' energy separation is in the microwave region, making it possible to drive hyperfine transitions using microwave radiation. However, at present no emitter is available that can be focused to address a particular ion from a sequence. Instead, a pair of laser pulses can be used to drive the transition, by having their frequency difference (detuning) equal to the required transition's frequency. This is essentially a stimulated Raman transition. References
See also
Categories: Atomic physics | Foundational quantum physics |
|
| This article is licensed under the GNU Free Documentation License. It uses material from the Wikipedia article "Hyperfine_structure". A list of authors is available in Wikipedia. |





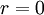 ).
).
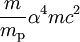
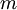 is the mass of an electron,
is the mass of an electron,
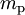 is the mass of a proton,
is the mass of a proton,
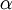 is the fine structure constant
is the fine structure constant 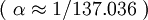 , and
, and
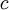 is the
is the 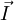 and the
and the 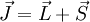 get coupled, giving rise to the total angular momentum
get coupled, giving rise to the total angular momentum 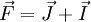 .
The hyperfine splitting is then
.
The hyperfine splitting is then
![\Delta E_{\rm hfs} = - \vec{\mu}_I \vec{B}_J = \frac{a}{2} [ F(F+1) - I(I+1) - J(J+1)],](images/math/1/c/7/1c7fcb57df311789e2be0ef2c30c6527.png)
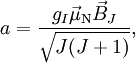
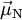 the
the 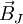 is the atomic magnetic field.
is the atomic magnetic field.
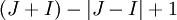 energy levels, where
energy levels, where 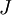 denotes the total electron angular momentum and
denotes the total electron angular momentum and 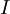 denotes the nuclear spin.
denotes the nuclear spin.
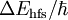 is of order of GHz; the hyperfine splitting is orders of magnitude smaller perturbation than the
is of order of GHz; the hyperfine splitting is orders of magnitude smaller perturbation than the 

