To use all functions of this page, please activate cookies in your browser.
my.chemeurope.com
With an accout for my.chemeurope.com you can always see everything at a glance – and you can configure your own website and individual newsletter.
- My watch list
- My saved searches
- My saved topics
- My newsletter
Isochron datingIsochron dating is a common technique of radiometric dating and is applied to date certain events, such as crystallization, metamorphism, shock events, and differentiation of precursor melts, in the history of rocks. Isochron dating can be further separated into mineral isochron dating and whole rock isochron dating; both techniques are applied frequently to date terrestrial and also extraterrestrial rocks (meteorites). The advantage of isochron dating as compared to simple radiometric dating techniques is that no assumptions about the initial amount of the daughter nuclide in the radioactive decay sequence are needed. Indeed the initial amount of the daughter product can be determined using isochron dating. This technique can be applied if the daughter element has at least one stable isotope other than the daughter isotope into which the parent nuclide decays. Product highlight
Basis for methodAll forms of isochron dating assume that the source of the rock or rocks contained unknown amounts of both radiogenic and non-radiogenic isotopes of the daughter element, along with some amount of the parent nuclide. Thus, at the moment of crystallization, the ratio of the concentration of the radiogenic isotope of the daughter element to that of the non-radiogenic isotope is some value independent of the concentration of the parent. As time goes on, some amount of the parent decays into the radiogenic isotope of the daughter, increasing the ratio of the concentration of the radiogenic isotope to that of the daughter. The greater the initial concentration of the parent, the greater the concentration of the radiogenic daughter isotope will be at some particular time. Thus, the ratio of the daughter to non-radiogenic isotope will become larger with time, while the ratio of parent to daughter will become smaller. For rocks that start out with a small concentration of the parent, the daughter/non-radiogenic ratio will not change quickly as compared to rocks starting with a large concentration of the parent. Isochron plotsTo perform mineral isochron dating, a rock is separated into several different minerals with different ratios between parent and daughter concentrations. For each mineral, the ratios are related by the following equation:
where
The proof of (1) amounts to simple algebraic manipulation. It is useful in this form because it exhibits the relationship between quantities that actually exist at present. To wit, P − ΔPt, D + ΔPt and Di respectively correspond to the concentrations of parent, daughter and non-radiogenic isotopes found in the rock at the time of measurement. The ratios If all data points lie on a straight line, this line is called an isochron. The better the fit of the data points to a line, the more reliable the resulting age estimate. Since the ratio of the daughter and non-radiogenic isotopes is proportional to the ratio of the parent and non-radiogenic isotopes, the slope of the isochron gets steeper with time. The slope of the isochron, Whole rock isochron dating uses the same ideas but instead of different minerals obtained from one rock uses different types of rocks that are derived from a common reservoir; e.g. the same precursor melt. It is possible to date the differentiation of the precursor melt which then cooled and crystallized into the different types of rocks. One of the best known isotopic systems for isochron dating is the rubidium-strontium system. Other systems that are used for isochron dating include samarium-neodymium, and uranium-lead. Some isotopic systems based on short living extinct radionuclides such as 53Mn, 27Al, 127Xe, 60Fe and others are used for isochron dating of events in the early history of the solar system. However, methods using extinct radionuclides give only relative ages and have to be calibrated with radiometric dating techniques based on long living radionuclides like Pb-Pb-dating to give absolute ages. ApplicationIsochron dating is useful in the determination of the age of igneous rocks, which have their initial origin in the cooling of liquid magma. It is also useful to determine the time of metamorphism, shock events (such as the consequence of an asteroid impact) and other events depending of the behaviour of the particular isotopic systems under such events. See also |
|
| This article is licensed under the GNU Free Documentation License. It uses material from the Wikipedia article "Isochron_dating". A list of authors is available in Wikipedia. |





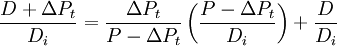 (1)
(1)
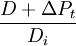 (relative concentration of daughter and non-radiogenic isotopes) and
(relative concentration of daughter and non-radiogenic isotopes) and 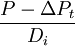 (relative concentration of parent and non-radiogenic isotope) are measured by
(relative concentration of parent and non-radiogenic isotope) are measured by 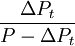 , represents the ratio of daughter to parent as used in standard
, represents the ratio of daughter to parent as used in standard 

