To use all functions of this page, please activate cookies in your browser.
my.chemeurope.com
With an accout for my.chemeurope.com you can always see everything at a glance – and you can configure your own website and individual newsletter.
- My watch list
- My saved searches
- My saved topics
- My newsletter
Photoacoustic imaging in biomedicine
Photoacoustic imaging, as a hybrid biomedical imaging modality, is developed based on the photoacoustic effect. In photoacoustic imaging, non-ionizing laser pulses are delivered into biological tissues (when radio frequency pulses are used, the technology is referred to as thermoacoustic imaging). Some of the delivered energy will be absorbed and converted into heat, leading to transient thermoelastic expansion and thus wideband (e.g. MHz) ultrasonic emission. The generated ultrasonic waves are then detected by ultrasonic transducers to form images. It is known that optical absorption is closely associated with physiological properties, such as hemoglobin concentration and oxygen saturation [2]. As a result, the magnitude of the ultrasonic emission (i.e. photoacoustic signal), which is proportional to the local energy deposition, reveals physiologically specific optical absorption contrast. 2D or 3D images of the targeted areas can then be formed [3]. Fig. 1 is a schematic illustration showing the basic principles of photoacoustic imaging.
Product highlight
Advantages of photoacoustic imaging
In functional imaging, optical imaging is highly desirable because of the strong correlation between optical absorption and hemoglobin concentration and/or oxygenation. However, existing high-resolution optical imaging technologies, including confocal microscopy, two-photon microscopy, and optical coherence tomography (OCT), do not sense optical absorption directly (Table 1). Moreover, since these imaging modalities rely on ballistic photons for imaging, their imaging depths are limited (Table 1) due to the strong optical scattering in biological tissues (OCT imaging in relatively transparent tissues such as the eye is an exception). Photoacoustic imaging, however, does not rely on ballistic photons for excitation; and ultrasonic waves have 2-3 orders of magnitude weaker scattering than optical waves in biological tissues. Consequently, photoacoustic imaging provides high resolution at relatively large imaging depth (Table 1). Similar to ultrasound imaging, the resolution and imaging depth of photoacoustic imaging is scalable, depending on the frequency of the ultrasound transducer used (refer to the section of photoacoustic microscopy (PAM) for details). Therefore, photoacoustic imaging combines the advantages of optical absorption contrast with ultrasonic spatial resolution for deep imaging beyond the ballistic regime [5]. Photoacoustic imaging systemsTwo types of photoacoustic imaging systems, photoacoustic/thermoacoustic computed tomography (also known as photoacoustic/thermoacoustic tomography, i.e., PAT/TAT) and photoacoustic microscopy (PAM), have been developed. A typical PAT system uses an unfocused ultrasound detector to acquire the photoacoustic signals, and the image is reconstructed by inversely solving the photoacoustic equations. A PAM system, on the other hand, uses a spherically focused ultrasound detector with 2D point-by-point scanning and requires no reconstruction algorithm.
Photoacoustic/thermoacoustic computed tomography (PAT/TAT)General photoacoustic equationGiven the heating function 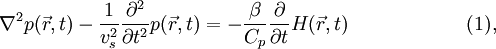 where vs is the speed of sound in medium, β is the thermal expansion coefficient, and Cp is the specific heat capacity at constant pressure. Eq. (1) holds under thermal confinement to ensure that heat conduction is negligible during the laser pulse excitation. The thermal confinement occurs when the laser pulsewidth is much shorter than the thermal relaxation time[5].
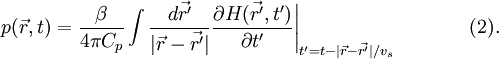
![p(\vec{r},t)=\frac{1}{4 \pi v_s^2} \frac{\partial}{\partial t} \left [\frac{1}{v_s t} \int d \vec{r'} p_0(\vec{r'}) \delta \left (t-\frac{|\vec{r}-\vec{r'}|}{v_s} \right) \right] \qquad \,(3),](images/math/4/3/7/4375ea56f55508e36b30cb4e804881e6.png) where p0 is the initial photoacoustic pressure. Universal reconstruction algorithmIn PAT system, the acoustic pressure is detected by scanning an ultrasound transducer over a surface that encloses the photoacoustic source. To reconstruct the internal source distribution, we need to solve the inverse problem of equation (3) (i.e. to obtain p0). A representative method applied for PAT reconstruction is known as the universal back projection algorithm[6]. This method is suitable for three imaging geometries: planar, spherical, and cylindrical surfaces. The universal backprojection formula is ![\left.p_0(\vec{r})=\int_{\Omega_0} \frac{d \Omega_0}{\Omega_0} \left [2 p(\vec{r_0},v_s t) - 2 v_s t \frac{\partial p(\vec{r_0},v_s t)}{\partial (v_s t)} \right]\right|_{t=|\vec{r} - \vec{r_0}|/v_s},\qquad \quad(4),](images/math/c/6/3/c63676289ecf30d3a8be4bd1ce57465f.png) where Ω0 is the solid angle subtended by the entire surface S0 with respect to the reconstruction point 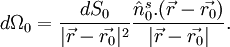 A typical PAT/TAT systemA typical PAT system is shown as the left part in Fig. 3. The laser beam is expanded and diffused to cover the whole region of interest. Photoacoustic waves are generated proportional to the distribution of optical absorption in the target, and are detected by an ultrasonic transducer. TAT system is the same as PAT system except using a microwave excitation source instead of laser. A typical TAT system is shown as the right part in Fig. 3. Although single-element transducers have been employed in these two systems, the detection scheme can be extended to using ultrasound arrays as well.
Biomedical applications of PAT/TATIntrinsic optical/microwave absorption contrast and diffraction-limited high spatial resolution of ultrasound make PAT and TAT promising imaging modalities for wide biomedical applications: Brain lesion detection[9]Soft tissues with different optical absorption properties in the brain can be clearly identified by PAT. For example, the absorption contrast between the lesion area and the background parenchyma is significant as shown in Fig. 4(a). Fig. 4(b) is the corresponding open-skull photograph after experiment.
Hemodynamics monitoring [7][9]
Since HbO2 and Hb are the dominant absorbing compounds in biological tissues in the visible spectral range, multiple wavelength photoacoustic measurements can be used to reveal the relative concentration of these two chromophores. Thus, the relative total concentration of hemoglobin (HbT) and the hemoglobin oxygen saturation (sO2) can be derived. Therefore, cerebral hemodynamic changes associated with brain function can be successfully detected with PAT.
Fig. 6 shows functional changes of sO2 and HbT in the rat cerebral cortex as a result of the physiological modulations. Under the hyperoxia status, the averaged sO2 level, Breast cancer diagnosis[10]By utilizing low scattered microwave for excitation, TAT is capable of penetrating thick (several cm) biological tissues with less than mm spatial resolution. Since the cancer tissue and normal tissue have very different response to radio frequency, TAT has great potential in early breast cancer diagnosis. Fig. 7 shows the TAT image of a mastectomy specimen, where malignant breast tissue generates a much stronger thermoacoustic signal than the surrounding benign tissue due to its high microwave absorption. Photoacoustic microscopy (PAM)Fig. 8 shows a representative PAM set-up [1]. A tunable dye laser is pumped by a Q-switched pulsed Nd:YAG (neodymium: yttrium aluminum garnet) laser. A short laser pulse at a certain wavelength between 532-770 nm is generated to irradiate the target tissue to induce acoustic pressure waves. Laser pulses of ~6 mJ/cm2 at the focus will be delivered at 10 Hz repetition rate. An Optical fiber of 0.6 mm core diameter is coaxially positioned on a three-dimensional mechanical stage with changeable ultrasound transducers between 25-75 MHz.
The imaging depth of PAM is mainly limited by the ultrasonic attenuation. The spatial and lateral resolutions depend on the ultrasound transducer used. An ultrasound transducer with high central frequency and broader bandwidth are chosen to obtain high axial resolution. The lateral resolution is determined by the focal diameter of the transducer. For instance, a 50-MHz ultrasound transducer provides 15 micron axial and 45 micron lateral resolution with ~3 mm imaging depth.
References
|
|
| This article is licensed under the GNU Free Documentation License. It uses material from the Wikipedia article "Photoacoustic_imaging_in_biomedicine". A list of authors is available in Wikipedia. |





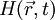 , the generation and propagation of photoacoustic wave pressure
, the generation and propagation of photoacoustic wave pressure 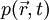 in an acoustically homogeneous inviscid medium is governed by
in an acoustically homogeneous inviscid medium is governed by
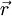 inside
inside 

