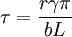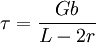To use all functions of this page, please activate cookies in your browser.
my.chemeurope.com
With an accout for my.chemeurope.com you can always see everything at a glance – and you can configure your own website and individual newsletter.
- My watch list
- My saved searches
- My saved topics
- My newsletter
Precipitation strengtheningPrecipitation hardening, also called age hardening or dispersion hardening, is a heat treatment technique used to strengthen malleable materials, including most structural alloys of aluminium, magnesium and titanium, and some stainless steels. It relies on changes in solid solubility with temperature to produce fine particles of an impurity phase, which impede the movement of dislocations, or defects in a crystal's lattice. Since dislocations are often the dominant carriers of plasticity, this serves to harden the material. The impurities, in fact, play the same role as matrix substances in composite materials. Just as the formation of ice in air can produce clouds, snow, or hail, depending upon the thermal history of a given portion of the atmosphere, precipitation in solids can produce many different sizes of particles, which have radically different properties. Unlike ordinary tempering, alloys must be kept at elevated temperature for hours to allow precipitation to take place. This time delay is called aging. Note that two different heat treatments involving precipitates can alter the strength of a material: solution heat treating and precipitation heat treating. Solution heat treating involves formation of a single-phase solid solution via quenching and leaves a material softer. Precipitation heat treating involves the addition of impurity particles to increase a material's strength.[1] Precipitation hardening via precipitation heat treatment is the main topic of discussion in this article. Product highlight
Kinetics versus thermodynamicsThis technique exploits the phenomenon of supersaturation, and involves careful balancing of the driving force for precipitation and the thermal activation energy available for both desirable and undesirable processes. Nucleation occurs at a relatively high temperature (often just below the solubility limit) so that the kinetic barrier of surface energy can be more easily overcome and the maximum number of precipitate particles can form. These particles are then allowed to grow at lower temperature in a process called aging. This is carried out under conditions of low solubility so that thermodynamics drive a greater total volume of precipitate formation. Diffusion's exponential dependence upon temperature makes precipitation strengthening, like all heat treatments, a fairly delicate process. Too little diffusion (under aging), and the particles will be too small to impede dislocations effectively; too much (over aging), and they will be too large and dispersed to interact with the majority of dislocations. Alloy designPrecipitation strengthening is possible if the line of solid solubility slopes strongly toward the center of a phase diagram. While a large volume of precipitate particles is desirable, little enough of the alloying element should be added that it remains easily soluble at some reasonable annealing temperature. Elements used for precipitation strengthening of typical aluminum and titanium alloys make up about 10% of their composition. While binary alloys are more easily understood as an academic exercise, commercial alloys often use three components for precipitation strengthening, in compositions such as Al(Mg, Cu) and Ti(Al, V). A large number of other constituents may be unintentional, but benign, or may be added for other purposes such as grain refinement or corrosion resistance. In some cases, such as many aluminum alloys, an increase in strength is achieved at the expense of corrosion resistance. The addition of large amounts of nickel and chromium needed for corrosion resistance in stainless steels means that traditional hardening and tempering methods are not effective. However, precipitates of chromium, copper or other elements can strengthen the steel by similar amounts to hardening and tempering. The strength can be tailored by adjusting the precipitation temperature, with lower temperatures resulting in higher strengths. Many alloy systems allow the aging temperature to be adjusted. For instance, some aluminium alloys used to make rivets for aircraft construction are kept in dry ice from their initial heat treatment until they are installed in the structure. After this type of rivet is deformed into its final shape, aging occurs at room temperature and increases its strength, locking the structure together. Higher aging temperatures would risk over-aging other parts of the structure, and require expensive post-assembly heat treatment. TheoryThe primary species of precipitation strengthening are second phase particles. These particles impede the movement of dislocations throughout the lattice. You can determine whether or not second phase particles will precipitate into solution from the solidus line on the phase diagram for the particles. Physically, this strengthening effect can be attributed both to size and modulus effects, and to interfacial or surface energy. The presence of second phase particles often causes lattice distortions. These lattice distortions result when the precipitate particles differ in size from the host atoms. Smaller precipitate particles in a host lattice leads to a tensile stress, whereas larger precipitate particles leads to a compressive stress. Dislocation defects also create a stress field. Above the dislocation there is a compressive stress and below there is a tensile stress. Consequently, there is a negative interaction energy between a dislocation and a precipitate that each respectively cause a compressive and a tensile stress or vice versa. In other words, the dislocation will be attracted to the precipitate. In addition, there is a positive interaction energy between a dislocation and a precipitate that have the same type of stress field. This means that the dislocation will be repulsed by the precipitate. Precipitate particles also serve by locally changing the stiffness of a material. Dislocations are repulsed by regions of higher stiffness. Conversely, if the precipitate causes the material to be locally more compliant, then the dislocation will be attracted to that region. Furthermore, a dislocation may cut through a precipitate particle. This interaction causes an increase in the surface area of the particle. The area created is where, r is the radius of the particle and b is the magnitude of the burgers vector. The resulting increase in surface energy is where Governing EquationsThere are two equations to describe the two mechanisms for precipitation hardening: Dislocations cutting through particles: where "tau" is material strength, "r" is the second phase particle radius, "gamma" is the surface energy, "b" is the magnitude of the Burgers vector, and "L" is the spacing between pinning points. This governing equation shows that the strength is proportional to r, the radius of the precipitate particles. This means that it is easier for dislocations to cut through a material with smaller second phase particles (small r). As the size of the second phase particles increases, the particles impede dislocation movement and it becomes increasingly difficult for the particles to cut through the material. In other words, the strength of a material increases with increasing r.
where "tau" is the material strength, "G" is the shear modulus, "b" is themagnitude of the Burgers vector, "L" is the distance between pinning points, and "r" is the second phase particle radius. This governing equation shows that for dislocation bowing the strength is inversely proportional to the second phase particle radius r. Dislocation bowing is more likely to occur when there are large particles present in the material.
These governing equations show that the precipitation hardening mechanism depends on the size of the precipitate particles. At small r, cutting will be the dominant strengthening mechanism, while at large r, bowing will be the dominant strengthening mechanism. Looking at the plot of both equations, it is clear that there is a critical radius at which max strengthening occurs. This critical radius is typically 5-30 nm. Some precipitation hardening materials
See also
Related reference
References
Categories: Metals processes | Materials science |
|
| This article is licensed under the GNU Free Documentation License. It uses material from the Wikipedia article "Precipitation_strengthening". A list of authors is available in Wikipedia. |





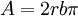
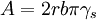
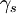 is the surface energy. The dislocation can also bow around
a precipitate particle.
is the surface energy. The dislocation can also bow around
a precipitate particle.