To use all functions of this page, please activate cookies in your browser.
my.chemeurope.com
With an accout for my.chemeurope.com you can always see everything at a glance – and you can configure your own website and individual newsletter.
- My watch list
- My saved searches
- My saved topics
- My newsletter
Psychrometric constantThe psychrometric constant, γ = , ralates the partial pressure of water in air to the air temperature. This lets one interpolate actual vapor pressure from paired dry and wet thermometer bulb temperature readings[1].
Product highlight
Although Thus on average, at a given location or altitude, the psychrometric constant is approximately constant. Still, it is worth remembering that weather impacts both atmospheric pressure and composition. vapor pressure estimationSaturated vapor pressure, 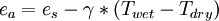
References
Categories: Chemical properties | Gas laws | Chemical engineering | Physical chemistry | Gases |
| This article is licensed under the GNU Free Documentation License. It uses material from the Wikipedia article "Psychrometric_constant". A list of authors is available in Wikipedia. |


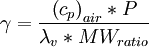



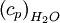 is constant, varied air composition results in varied
is constant, varied air composition results in varied 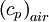 .
.
![e_s = e \left[ T_{dew}\right] = e \left[ T_{wet} \right]](images/math/9/a/5/9a5c326ecdb74394f42e90b15ed5a65d.png)


