To use all functions of this page, please activate cookies in your browser.
my.chemeurope.com
With an accout for my.chemeurope.com you can always see everything at a glance – and you can configure your own website and individual newsletter.
- My watch list
- My saved searches
- My saved topics
- My newsletter
Atmospheric pressureAtmospheric pressure is the pressure at any point in the Earth's atmosphere. In most circumstances atmospheric pressure is closely approximated by the hydrostatic pressure caused by the weight of air above the measurement point. Low pressure areas have less atmospheric mass above their location, whereas high pressure areas have more atmospheric mass above their location. Similarly, as elevation increases there is less overlying atmospheric mass, so that pressure decreases with increasing elevation. A column of air 1 square inch in cross section, measured from sea level to the top of the atmosphere, would weigh approximately 14.7 lbf. A 1 m² (11 sq ft) column of air would weigh about 100 kilonewtons (equivalent to a mass of 10.2 tonnes at the surface). Product highlightStandard atmospheric pressureThe standard atmosphere (symbol: atm) is a unit of pressure and is defined as being precisely equal to 101.325 kPa. The following non-standard units are equivalent: 760 mmHg (torr) or 29.92 inHg. One standard atmosphere is standard pressure used for pneumatic fluid power (ISO R554), and in the aerospace (ISO 2533) and petroleum (ISO 5024) industries. In 1999, the International Union of Pure and Applied Chemistry (IUPAC) recommended that for the purposes of specifying the physical properties of substances, “the standard pressure” should be defined as precisely 100 kPa (≈750.01 torr) or 29.53 inHg rather than the 101.325 kPa value of “one standard atmosphere”.[1] This value is used as the standard pressure for the compressor and the pneumatic tool industries (ISO 2787).[2] (see also Standard temperature and pressure) In the United States, compressed air flow is often measured in "standard cubic yards" per unit of time, where the "standard" means the equivalent quantity of moisture at standard temperature and pressure. However, this standard atmosphere is defined slightly differently: temperature = 20 °C (68 °F), air density = 1.225 kg/m³ (0.0765 lb/cu ft), altitude = sea level, and relative humidity = 20%. In the air conditioning industry, the standard is often temperature = 0 °C (32 °F) instead. For natural gas, the petroleum industry uses a standard temperature of 15.6 °C (60.08 °F), pressure 101.56 kPa (14.73 psi). Mean sea level pressure
Mean sea level pressure (MSLP or QFF) is the pressure at sea level or (when measured at a given elevation on land) the station pressure reduced to sea level assuming an isothermal layer at the station temperature. This is the pressure normally given in weather reports on radio, television, and newspapers or on the Internet. When barometers in the home are set to match the local weather reports, they measure pressure reduced to sea level, not the actual local atmospheric pressure. See Altimeter (barometer vs. absolute). The reduction to sea level means that the normal range of fluctuations in pressure is the same for everyone. The pressures which are considered high pressure or low pressure do not depend on geographical location. This makes isobars on a weather map meaningful and useful tools. The altimeter setting in aviation, set either QNH or QFE, is another atmospheric pressure reduced to sea level, but the method of making this reduction differs slightly. See altimeter.
QFE and QNH are arbitary Q codes rather than abbreviations, but the mnemonics "Nautical Height" (for QNH) and "Field Elevation" (for QFE) are often used by pilots to distinguish them. Average sea-level pressure is 101.325 kPa (1013.25 mbar) or 29.921 inches of mercury (inHg) or 760 millimeters (mmHg). In aviation weather reports (METAR), QNH is transmitted around the world in millibars or hectopascals (1 millibar = 1 hectopascal), except in the United States and in Canada where it is reported in inches (or hundredths of inches) of mercury. (The United States and Canada also report sea level pressure SLP, which is reduced to sea level by a different method, in the remarks section, not an internationally transmitted part of the code, in hectopascals or millibars [3]. However, in Canada's public weather reports, sea level pressure is instead reported in kilopascals [1], while Environment Canada's standard unit of pressure is the same [2] [3].) In the weather code, three digits are all that is needed; decimal points and the one or two most significant digits are omitted: 1013.2 mbar or 101.32 kPa is transmitted as 132; 1000.0 mbar or 100.00 kPa is transmitted as 000; 998.7 mbar or 99.87 kPa is transmitted as 987; etc. The highest sea-level pressure on Earth occurs in Siberia, where the Siberian High often attains a sea-level pressure above 1032.0 mbar. The lowest measurable sea-level pressure is found at the centers of hurricanes (typhoons, baguios) Altitude atmospheric pressure variationPressure varies smoothly from the earth's surface to the top of the mesosphere. Although the pressure changes with the weather, NASA has averaged the conditions for all parts of the earth year-round. The following is a list of air pressures (as a fraction of one atmosphere) with the corresponding average altitudes. The table gives a rough idea of air pressure at various altitudes.
Calculating variation with altitude
There are two different equations for computing pressure at various height regimes below 86 km (or 278,400 ft). Equation 1 is used when the value of standard temperature lapse rate is not equal to zero and equation 2 is used when standard temperature lapse rate equals zero. Equation 1: Equation 2: where
Or converted to English units:[4] where
The value of subscript b ranges from 0 to 6 in accordance with each of seven successive layers of the atmosphere shown in the table below. In these equations, g0, M and R* are each single-valued constants, while P, L, T, and h are multivalued constants in accordance with the table below. It should be noted that the values used for M, g0, and R * are in accordance with the U.S. Standard Atmosphere, 1976, and that the value for R * in particular does not agree with standard values for this constant.[5] The reference value for Pb for b = 0 is the defined sea level value, P0 = 101325 pascals or 29.92126 inHg. Values of Pb of b = 1 through b = 6 are obtained from the application of the appropriate member of the pair equations 1 and 2 for the case when h = hb + 1.:[5]
Sample Calculation:Find the pressure at 30,000 meters. First note that 30,000 meters is above 20,000 but below 32,000 so it therefore falls in the range of subscript b=2 in the chart above. Also note that the temperature lapse rate for that region is not equal to zero; therefore, equation 1 is appropriate. Or
Local atmospheric pressure variation
Atmospheric pressure varies widely on Earth, and these changes are important in studying weather and climate. See pressure system for the effects of air pressure variations on weather. The highest recorded atmospheric pressure, 108.6 kPa (1,086 mbar or 32.06 inches of mercury), occurred at Tosontsengel, Khövsgöl Province, Mongolia, 19 December, 2001.2[not in citation given] The lowest recorded non-tornadic atmospheric pressure, 87.0 kPa (870 mbar or 25.69 inches of mercury), occurred in the Western Pacific during Typhoon Tip on 12 October, 1979.2[not in citation given] The record for the Atlantic ocean was 88.2 kPa (882 mbar or 26.04 inches of mercury) during Hurricane Wilma on 19 October 2005. Atmospheric pressure shows a diurnal (twice-daily) cycle caused by global atmospheric tides. This effect is strongest in tropical zones, with amplitude of a few millibars, and almost zero in polar areas. A graph on the top of this page shows these rhythmic variations in northern Europe. These variations have two superimposed cycles, a circadian (24 h) cycle and semi-circadian (12 h) cycle. Atmospheric pressure based on height of waterAtmospheric pressure is often measured with a mercury barometer, and a height of approximately 760 mm (30 inches) of mercury is often used to teach, make visible, and illustrate (and measure) atmospheric pressure. However, since mercury is not a substance that humans commonly come in contact with, water often provides a more intuitive way to conceptualize the amount of pressure in one atmosphere. One atmosphere (101.325 kPa or 14.7 lbf/sq in) is the amount of pressure that can lift water approximately 10.3 m (33.9 ft). Thus, a diver at a depth 10.3 meters under water in a fresh-water lake experiences a pressure of about 2 atmospheres (1 atm for the air and 1 atm for the water). This is also the maximum height to which a column of water can be drawn up by suction. Non-professional barometers are generally aneroid barometer (Figure 3) or strain gauge based. See Pressure measurement for a description of barometers. Atmospheric pressure's relation to water's boiling pointAlthough water is generally considered to boil at 100° C (212° F), water actually evaporates when the vapor pressure is equal to the atmospheric pressure around the water. [6] Because of this, the boiling point of water is decreased in lower pressure and raised at higher pressure. This is why baking at altitudes beyond 3,500 feet above sea level requires special baking directions. [7] See also
References
Experiments
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| This article is licensed under the GNU Free Documentation License. It uses material from the Wikipedia article "Atmospheric_pressure". A list of authors is available in Wikipedia. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||





![{P}=P_b \cdot \left[\frac{T_b}{T_b + L_b\cdot(h-h_b)}\right]^\frac{g_0 \cdot M}{R^* \cdot L_b}](images/math/e/5/c/e5c63528923d557f1c1215c3257ff111.png)
![{P}=P_b \cdot \exp \left[\frac{-g_0 \cdot M \cdot (h-h_b)}{R^* \cdot T_b}\right]](images/math/4/4/6/4462d97ee83b9422618bb06c1bd9c46c.png)
![{P}=P_2 \cdot \left[\frac{T_2}{T_2 + L_2\cdot(h-h_2)}\right]^\frac{g_0 \cdot M}{R^* \cdot L_2}](images/math/9/e/1/9e147b304976c9f310f96fed520b6348.png)
![{P}=5474.89 \cdot \left[\frac{216.65}{216.65 + 0.001\cdot(30,000-20,000)}\right]^\frac{9.80665 \cdot 28.9644}{8314.32 \cdot 0.001}](images/math/9/0/5/905aa4dcf24dca9dce20eb519f1484a0.png)
![{P}=5474.89 \cdot \left[\frac{216.65}{226.65}\right]^{34.163195}](images/math/4/c/1/4c18860885f24522073c05f97d8b0beb.png)
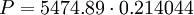
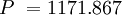 Pascals at 30,000 meters
Pascals at 30,000 meters


