To use all functions of this page, please activate cookies in your browser.
my.chemeurope.com
With an accout for my.chemeurope.com you can always see everything at a glance – and you can configure your own website and individual newsletter.
- My watch list
- My saved searches
- My saved topics
- My newsletter
Standard enthalpy change of combustionThe standard enthalpy of combustion is the enthalpy change when one mole of a substance completely reacts with oxygen under standard thermodynamic conditions (although experimental values are usually obtained under different conditions and subsequently adjusted). By definition, combustion reactions are generally strongly exothermic and so enthalpies of combustion are generally strongly negative. Product highlightIt is commonly denoted as Enthalpies of combustion are typically measured using bomb calorimetry, and have units of energy (typically kJ); strictly speaking, the enthalpy change per mole of substance combusted is the standard molar enthalpy of combustion (which typically would have units of kJ mol−1). |
| This article is licensed under the GNU Free Documentation License. It uses material from the Wikipedia article "Standard_enthalpy_change_of_combustion". A list of authors is available in Wikipedia. |





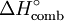 or
or