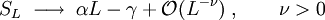To use all functions of this page, please activate cookies in your browser.
my.chemeurope.com
With an accout for my.chemeurope.com you can always see everything at a glance – and you can configure your own website and individual newsletter.
- My watch list
- My saved searches
- My saved topics
- My newsletter
Topological entropy (in physics)The topological entanglement entropy, usually denoted by γ, is a number characterizing many-particle states that possess topological order. The short form topological entropy is often used, although the same name in ergodic theory refers to an unrelated mathematical concept (see topological entropy). Product highlightGiven a topologically ordered state, the topological entropy can be extracted from the asymptotic behavior of the Von Neumann entropy measuring the quantum entanglement between a spatial block and the rest of the system. The entanglement entropy of a simply connected region of boundary length L, within an infinite two-dimensional topologically ordered state, has the following form for large L: The subleading constant term is the topological entanglement entropy. The topological entanglement entropy is equal to the logarithm of the total quantum dimension of the quasiparticle excitations of the state. For example, the simplest fractional quantum Hall states, the Laughlin states at filling fraction 1/m, have γ = ½log(m). The Z2 fractionalized states, such as topologically ordered states of quantum dimer models on non-bipartite lattices and Kitaev's toric code state, are characterized γ = log(2).
ReferencesIntroduction of the quantity:
Calculations for specific topologically ordered states:
Categories: Condensed matter physics | Statistical mechanics |
| This article is licensed under the GNU Free Documentation License. It uses material from the Wikipedia article "Topological_entropy_(in_physics)". A list of authors is available in Wikipedia. |