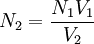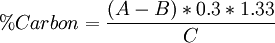To use all functions of this page, please activate cookies in your browser.
my.chemeurope.com
With an accout for my.chemeurope.com you can always see everything at a glance – and you can configure your own website and individual newsletter.
- My watch list
- My saved searches
- My saved topics
- My newsletter
Total organic carbonTotal organic carbon (TOC) is the amount of carbon bound in an organic compound and is often used as a non-specific indicator of water quality or cleanliness of pharmaceutical manufacturing equipment. A typical analysis for TOC measures both the total carbon present as well as the inorganic carbon (IC). Subtracting the inorganic carbon from the total carbon yields TOC. Another common variant of TOC analysis involves removing the IC portion first and then measuring the leftover carbon. This method involves purging an acidified sample with carbon-free air or nitrogen prior to measurement, and so is more accurately called non-purgeable organic carbon (NPOC). Product highlight
TOC AnalysisEnvironmentalTOC has a long history as being a world-renowned analytical technique to measure water quality during the drinking water purification process. TOC in source waters comes from decaying natural organic matter (NOM) and from synthetic sources. Humic acid, fuvic acid, amines, and urea are types of NOM. Detergents, pesticides, fertilizers, herbicides, industrial chemicals, and chlorinated organics are examples of synthetic sources. Before source water is treated for disinfection, TOC provides an important role in quantifying the amount of NOM in the water source. In water treatment facilities, source water is subject to reaction with chloride containing disinfectants. When the raw water is chlorinated, active chlorine compounds (Cl2, HOCl, ClO-) react with NOM to produce chlorinated disinfection byproducts (DBPs). Many researchers have determined that higher levels of NOM in source water during the disinfection process will increase the amount of carcinogens in the processed drinking water. In the 1970’s TOC analysis emerged as a rapid and accurate alternative to the classical but lengthy biological oxygen demand (BOD) and chemical oxygen demand (COD) tests traditionally reserved for assessing the pollution potential of wastewaters. Today, Environmental Protection Agencies regulate the trace limits of DBPs in drinking water. Recent methods, such as USEPA method 415.3, D/DBP rule, regulate the amount of NOM to prevent the formation of DBPs in finished waters. PharmaceuticalIntroduction of organic matter into water systems occurs only from living organisms and from decaying matter in source water, but also from purification and distribution system materials. A relationship may exist between endotoxins, microbial growth, and the development of biofilms on pipeline walls and biofilm growth within pharmaceutical distribution systems. A correlation is believed to exist between TOC concentrations and the levels of endotoxins and microbes. Sustaining high TOC levels helps to control levels of endotoxins and microbes and thereby the development of biofilm growth. The United States Pharmacopoeia (USP), European Pharmacopoeia (EP) and Japanese Pharmacopoeia (JP) recognize TOC as a required test for purified water and water for injection (WFI). For this reason, TOC has found acceptance as a process control attribute in the biotechnology industry to monitor the performance of unit operations comprising purification and distribution systems. As many of these biotechnology operations include the preparation of medicines, the Food, Drug Administration (FDA) enacts numerous regulations to protect the health of the public and ensure the product quality is maintained. To make sure there is no cross contamination between product runs of different drugs various cleaning procedures are performed. TOC concentration levels are used to track the success of these cleaning validation procedures especially clean-in-place (CIP). MeasurementTo understand the analysis process better, some key basic terminologies should be understood and their relationships to one another (Figure 1). Total Carbon (TC) – all the carbon in the sample, including both inorganic and organic carbon Total Inorganic Carbon (TIC) – often referred to as inorganic carbon (IC), carbonate, bicarbonate, and dissolved carbon dioxide; a material derived from non-living sources. Total Organic Carbon (TOC) – material derived from decaying vegetation, bacterial growth, and metabolic activities of living organisms or chemicals. Non-Purgeable Organic Carbon (NPOC) – commonly referred to as TOC; organic carbon remaining in a sample after purging the sample with gas. Purgeable (volatile) Organic Carbon (POC) – organic carbon that has been sparged or removed from a sample. Dissolved Organic Carbon (DOC) – organic carbon remaining in a sample after filtering the sample, typically using a 0.45 mm filter. Suspended Organic Carbon – also called particulate organic carbon (PtOC); the carbon in particulate form that is too large to pass through a filter.
There are two methodologies to perform TOC analysis. The first is a two-stage process commonly referred to as TC-IC. It measures both the amount of IC present from an acidified aliquot and the amount of TC present separately. The IC present in the sample is determined by lowering its pH to a value of two or less with acid. This liberates the IC from the sample in gaseous form. Then a hydrocarbon free carrier gas sparges through the sample sending the IC gas through the instrument removing unwanted water vapor and possible interfering substances before reaching the detector for measurement. The separate TC measurements involve no acidification only treatment to oxidize the carbon which causes the release of the gases within the sample, which are also sent through the instrument removing unwanted water vapor and possible interfering substances before reaching the detector for measurement. The resultant TOC value is a product of subtraction of the IC value from the TC the sample. The second more common method directly measures TOC in the sample by again acidifying the sample it to a pH value of two or less to release the IC gas but in this case to the air not for measurement. The remaining non-purgeable CO2 gas contained in the liquid aliquot is then oxidized releasing the gases. These gases are then sent to the detector for measurement. Whether the analysis of TOC is by TC-IC or NPOC methods, it may be broken into three main stages:
The first stage is acidification of the sample for the removal of the IC and POC gases. The release of these gases to the detector for measurement or to the air is dependent upon which type of analysis is of interest, the former for TC-IC and the latter for TOC (NPOC). AcidificationThe removal and venting of IC and POC gases from the liquid sample by acidification and sparging occurs in the following manner.
OxidationThe second stage is the oxidation of the carbon in the remaining sample in the form of carbon dioxide (CO2) and other gases. Modern TOC analyzers perform this oxidation step by one several processes:
HTCWith the combustion technique, a manual or automated process injects the sample into a furnace at a temperature between 680o - 900o C. The furnace contains a combustion tube packed with catalyst usually made of platinum and alumina. But the catalyst is a bad method to fulfil the combustion because salt fils the pores and fast reduces the activity of the catalyst. So thess temperatures are not always enough to have a full combustion of your injected sample. Therefore some systems work with a temperature of 850°C -1200°C, this way they have a full combustion of the sample. The salt will become gas and can be filtered out through a much cheaper scrubber. A good example of such a system is the combined HTC-UV TOC analyser from Thermo Scientific.
Oxidation of the sample is complete after injection into the furnace, turning the sample into gaseous form. A carbon-free carrier gas transports the gas, now in the form CO2, through the instrument. Within the sample path flow, a moisture trap and halide scrubbers remove of water vapor and halides from the gas stream before it reaches the detector. These substances can interfere with the detection of the CO2 gas. The HTC method may be useful in those applications where difficult to oxidize compounds are present as it provides excellent oxidation of organics and accommodates the analysis of solids and particulates. The major drawback of HTC analysis is its unstable baseline levels, which limits its usefulness for ultra-pure water analysis. This is due to the accumulation of nonvolatile residues within the combustion tube located in the high temperature reactor. These residues add false and continuously changing “TOC” background levels to the current TOC analysis, resulting in unreliable data. HTC analyzers generally exhibit low sensitivity and require high maintenance of the its high temperature components. Photo-Oxidation (UV Light)In this oxidation scheme, ultra-violet light alone oxidizes the carbon within the sample to produce CO2. The UV oxidation method offers the most reliable, low maintenance method of analyzing TOC in ultra-pure waters. The UV/Chemical (Persulfate) OxidationLike the photo-oxidation method, UV light is the oxidizer but the oxidation power of the reaction is magnified by the addition of a chemical oxidizer, which usually a persulfate compound. The mechanisms of the reactions are as follows:
The UV/chemical oxidation method offers a relatively low maintenance, high sensitivity method for a wide range of applications. However, there are oxidation limitations of this method. Limitations include the inaccuracies associated with the addition of any foreign substance into the analyte and samples with high amounts of particulates. By performing "System Blanks", which is to analyze then subtract the amount of carbon contributed by the chemical additive, helps lower inaccuracies but analyses in levels below 200ppb TOC are still difficult. Detection and QuantificationAccurate detection and quantification are the most vital components of the TOC analysis process. Conductivity and non-dispersive infrared (NDIR) are the two common detection methods used in modern TOC analyzers. ConductivityThere are two types of conductivity detectors, direct and membrane. Direct conductivity provides an inexpensive and simple means of measuring CO2. This method has good oxidation of organics, uses no carrier gas, is good at the parts per billion (ppb) ranges, but has a very limited analytical range. Membrane conductivity relies upon the same technology as direct conductivity. Although it is more robust than direct conductivity, it suffers from slow analysis time. Both methods analyze sample conductivity before and after oxidization, attributing this differential measurement to the TOC of the sample. During the sample oxidization phase, CO2 (directly related to the TOC in the sample) and other gases are formed. The dissolved CO2 forms a weak acid, thereby changing the conductivity of the original sample proportionately to the TOC in the sample. Conductivity analyses assume that only CO2 is present within the solution. As long as this holds true, then the TOC calculation by this differential measurement is valid. However, depending on the chemical species present in the sample and their individual products of oxidation, they may present either a positive or a negative interference to the actual TOC value, resulting in analytical error. Some of the interfering chemical species include Cl-, HCO3-, SO32-, SO2-, ClO2-, and H+. Small changes in pH and temperature fluctuations also contribute to inaccuracy. Membrane conductivity analyzers have tried to improve upon the direct conductivity approach by incorporating the use of hydrophobic gas permeation membranes to allow a more “selective” passage of the dissolved CO2 gas. While this has solved certain problems, membranes have their own particular limitations, such as with true selectivity, clogging and, more undetectably, they provide secondary sites for other chemical reactions, which are prone to display “false negatives,” a condition far more severe than “false positives” in critical applications. Micro leaks, flow problems, dead spots, microbial growth (blockage) are also potential problems. Most disconcerting is the inability of membrane methods to recover to operational performance after an overload or “spill” condition arises to over range the instrument, often taking hours before returning to reliable service and recalibration, just when accuracy of TOC analysis is most critical to operators for quality control. Non-dispersive infrared (NDIR)The non-dispersive infrared analysis (NDIR) method offers the only practical interference-free method for detecting CO2 in TOC analysis. The principal advantage of using NDIR is that it directly and specifically measures the CO2 generated by oxidation of the organic carbon in the oxidation reactor, rather than relying on a measurement of a secondary, corrected effect, such as used in conductivity measurements. A traditional NDIR detector relies upon flow-through-cell technology, the oxidation product flows into and out of the detector continuously. A region of adsorption of infrared light specific to CO2, usually around 4.26 mm (2350 cm-1), is measured over time as the gas flows through the detector. The infrared adsorption spectra of CO2 and other gases is shown in Figure 3. A second reference measurement that is non-specific to CO2 is also taken and the differential result correlates to the CO2 concentration in the detector at that moment. As the gas continues to flow into an out of the detector cell the sum of the measurements results in a peak that is integrated and correlated to the total CO2 concentration in the sample aliquot.
Recent Advances in NDIR Technology The exit valve of the NDIR is closed to allow the detector to become pressurized. Once the gases in the detector have reached equilibrium, the concentration of the CO2 is analyzed. This pressurization of the sample gas stream in the NDIR, a patent-pending technique, allows for increased sensitivity and precision by measuring the entirety of the oxidation products of the sample in one reading, compared to flow-through cell technology. The output signal is proportional to the concentration of CO2 in the carrier gas, from the oxidation of the sample aliquot. UV/ Persulfate oxidation combined with NDIR detection provides good oxidation of organics, low instrument maintenance, good precision at ppb levels, relatively fast sample analysis time and easily accommodates multiple applications, including purified water (PW), water for injection (WFI), CIP, drinking water and ultra-pure water analyses.
AnalyzersVirtually all TOC analyzers measure the CO2 formed when organic carbon is oxidized and/or when inorganic carbon is acidified. Oxidation is performed either through Pt-catalyzed combustion or with a UV/persulfate reactor. Once the CO2 is formed, it is measured by a detector: either a conductivity cell (if the CO2 is aqueous) or a non-dispersive infrared cell (after purging the aqueous CO2 into the gaseous phase). Conductivity detection is only desirable in the lower TOC ranges in deionized waters, whereas NDIR detection excels in the higher TOC ranges. A variation described as Membrane Conductivity Detection can allow for measurement of TOC across a wide analytical range in both deionized and non-deionized water samples. Modern high-performance TOC instruments are capable of detecting carbon concentrations well below 1 µg/L (1 part per billion or ppb). A total organic carbon analyzer determines the amount of carbon in a water sample. By acidifying the sample and flushing with nitrogen or helium the sample removes inorganic carbon, leaving only organic carbon sources for measurement. There are two types of analyzers. One uses combustion and the other wet oxidation. This is used as a water purity test, as the presence of bacteria introduces organic carbon. CombustionIn a combustion analyzer, half the sample is injected into a chamber where it is acidified, usually with phosphoric acid, to turn all of the inorganic carbon into carbon dioxide as per the following reaction: CO2 + H2O ↔ H2CO3 ↔ H+ + HCO3- ↔ 2H+ + CO32- This is then sent to a detector for measurement. The other half of the sample is injected into a combustion chamber which is raised to between 600–700°C, some even up to 1200°C. Here, all the carbon reacts with oxygen, forming carbon dioxide. It's then flushed into a cooling chamber, and finally into the detector. Usually, the detector used is a non-dispersive infrared spectrophotometer. By finding the total inorganic carbon and subtracting it from the total carbon content, the amount of organic carbon is determined. Wet OxidationWet oxidation analyzers inject the sample into a chamber with ammonium peroxydisulfate and phosphoric acid. This is separated into two portions, one that goes into a delay coil which takes the inorganic carbon for measurement, using the same method as the combustion analyzers. The other half of the sample is injected into an oxidation chamber, where it is bombarded with UV light from a mercury vapor lamp. Here, free radicals form from the peroxydisulfate and react with any carbon available to form carbon dioxide. The carbon from both chambers are run through membranes which measure the conductivity changes that result from the presence of varying amounts of carbon dioxide. Same as the combustion analyzer, the total carbon formed minus the inorganic carbon gives a good estimate of the total organic carbon in the sample. This method is often used in online applications because of it's low maintenance requirements. For example the online Biotector witch is the most modern application of this method. ApplicationsTOC is the first chemical analysis to be carried out on potential petroleum source rock in oil exploration. It is very important in detecting contaminants in drinking water, cooling water, water used in semiconductor manufacturing, and water for pharmaceutical use. Analysis may be made either as an online continuous measurement or a lab-based measurement. TOC detection is an important measurement because of the effects it may have on the environment, human health, and manufacturing processes. TOC is a highly sensitive, non-specific measurement of all organics present in a sample. It, therefore, can be used to regulate the organic chemical discharge to the environment in a manufacturing plant. In addition, low TOC can confirm the absence of potentially harmful organic chemicals in water used to manufacture pharmaceutical products. TOC is also of interest in the field of potable water purification due to disinfection of byproducts. Inorganic carbon poses little to no threat. Walkley-Back modified methodCalculationIn the Total Organic Carbon determination to obtain the percent carbon content from soil, first we have to standardize the titrant solution (FeSO4•7H2O) before the sample analysis are made, as result we obtain some data which have to be reduced in order to obtain the results we need, and to do this, we use the next equations: Titrant normality equation: Eq.1 where: Organic carbon percentage: Eq.2 where:
The 0.3 conversion factor has units of carbon grams and involves the constant to convert a fraction to percent units; hence equation 2 does not have the factor 100. Walkey-Black constant for sediments. 75% is the mean recuperation of carbon in solids and sediments by using this method, thats why the final result has to be multiplied by 1.33 in order to get the real value, this constant is not used when determining carbon in KHP standard because almost all its carbon content is recovered. References
Categories: Chemical oceanography | Analytical chemistry |
|
| This article is licensed under the GNU Free Documentation License. It uses material from the Wikipedia article "Total_organic_carbon". A list of authors is available in Wikipedia. |