To use all functions of this page, please activate cookies in your browser.
my.chemeurope.com
With an accout for my.chemeurope.com you can always see everything at a glance – and you can configure your own website and individual newsletter.
- My watch list
- My saved searches
- My saved topics
- My newsletter
Transition dipole momentThe Transition dipole moment or Transition moment, usually denoted Product highlight
DefinitionThe transition dipole moment for the
where the summations are over the positions of the electrons in the system. Giving the transition dipole moment:
where the integral is, in principle over all space, but can be restricted to the region in which the initial and final state wavefunctions are non-negligible. Analogy with a classical dipoleA basic, phenomenological understanding of the transition dipole moment can be obtained by analogy with a classical dipole. While the comparison can be very useful, care must be taken to ensure that one does not fall into the trap of assuming they are the same. In the case of two classical point charges,
In the presence of an electric field, such as that due to an electromagnetic wave, the two charges will experience a force in opposite directions, leading to a net torque on the dipole. The magnitude of the torque is proportional to the magnitude of the charge, the separation and varies with the relative angles of the field and the dipole,
Similarly, the coupling between an electromagnetic wave and an atomic transition with transition dipole moment OriginWhen an atom or molecule interacts with an electromagnetic wave of frequency ApplicationsThe transition dipole moment is useful for determining if transitions are allowed. For example, the transition from a bonding ReferencesIUPAC compendium of Chemical Terminology. IUPAC (1997). Retrieved on 2007-01-15. |
|
| This article is licensed under the GNU Free Documentation License. It uses material from the Wikipedia article "Transition_dipole_moment". A list of authors is available in Wikipedia. |


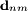 for a transition between an initial state,
for a transition between an initial state, 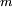 , and a final state,
, and a final state,  , is the electric dipole moment associated with the transition between the two states. In general the transition dipole moment is a complex vector quantity that includes the phase factors associated with the two states. Its direction gives the polarization of the transition, which determines how the system will interact with an electromagnetic wave of a given polarization, while the square of the magnitude gives the strength of the interaction due to the distribution of charge within the system. The
, is the electric dipole moment associated with the transition between the two states. In general the transition dipole moment is a complex vector quantity that includes the phase factors associated with the two states. Its direction gives the polarization of the transition, which determines how the system will interact with an electromagnetic wave of a given polarization, while the square of the magnitude gives the strength of the interaction due to the distribution of charge within the system. The 


 transition is given by the relevant off-diagonal element of the dipole matrix, which can be calculated from an integral taken over the product of the wavefunctions of the initial and final states of the transition, and the dipole moment operator,
transition is given by the relevant off-diagonal element of the dipole matrix, which can be calculated from an integral taken over the product of the wavefunctions of the initial and final states of the transition, and the dipole moment operator,
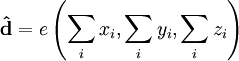 ,
,
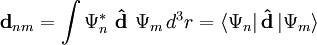 ,
,
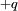 and
and 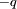 , with a displacement vector,
, with a displacement vector,  , pointing from the negative charge to the positive charge, the electric dipole moment is given by
, pointing from the negative charge to the positive charge, the electric dipole moment is given by
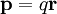 .
.
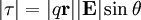 .
.
 , it can undergo a transition to a higher energy state by absorbing a
, it can undergo a transition to a higher energy state by absorbing a 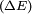 is the same as the photon energy
is the same as the photon energy 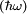 . The presence of the electromagnetic field can induce an oscillating electric dipole moment, referred to as the transition dipole moment. If the charge,
. The presence of the electromagnetic field can induce an oscillating electric dipole moment, referred to as the transition dipole moment. If the charge,  , is omitted one obtains
, is omitted one obtains 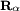 as used in
as used in  orbital to an antibonding
orbital to an antibonding 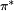 orbital is allowed because the integral defining the transition dipole moment is nonzero. Such a transition occurs between an even and an odd orbital; the dipole operator is an odd function of
orbital is allowed because the integral defining the transition dipole moment is nonzero. Such a transition occurs between an even and an odd orbital; the dipole operator is an odd function of 

