To use all functions of this page, please activate cookies in your browser.
my.chemeurope.com
With an accout for my.chemeurope.com you can always see everything at a glance – and you can configure your own website and individual newsletter.
- My watch list
- My saved searches
- My saved topics
- My newsletter
Weight average molecular weightThe weight average molecular weight is a way of describing the molecular weight of a polymer. Polymer molecules, even if of the same type, come in different sizes (chain lengths, for linear polymers), so we have to take an average of some kind. For the weight average molecular weight, this is calculated by Product highlight
where Ni is the number of molecules of molecular weight Mi. Intuitively, if the weight average molecular weight is w, and you pick a random monomer, then the polymer it belongs to will have a weight of w on average. The weight average molecular weight can be determined by light scattering, small angle neutron scattering (SANS), X-ray scattering, and sedimentation velocity. An alternative measure of molecular weight for a polymer is the number average molecular weight; the ratio of the weight average to the number average is called the polydispersity index. The weight-average molecular weight, Mw, is also related to the fractional monomer conversion, p, in step-growth polymerization as per Carothers' equation:
|
| This article is licensed under the GNU Free Documentation License. It uses material from the Wikipedia article "Weight_average_molecular_weight". A list of authors is available in Wikipedia. |





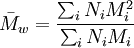
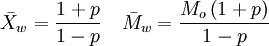 , where Mo is the molecular weight of the repeating unit.
, where Mo is the molecular weight of the repeating unit.


