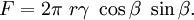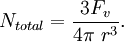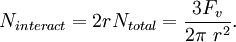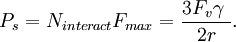To use all functions of this page, please activate cookies in your browser.
my.chemeurope.com
With an accout for my.chemeurope.com you can always see everything at a glance – and you can configure your own website and individual newsletter.
- My watch list
- My saved searches
- My saved topics
- My newsletter
Zener pinningZener pinning is the influence of a dispersion of fine particles on the movement of low- and high angle grain boundaries through a polycrystalline material. Small particles act to prevent the motion of such boundaries by exerting a pinning pressure which counteracts the driving force pushing the boundaries. Zener pinning is very important in materials processing as it has a strong influence on recovery, recrystallization and grain growth. Product highlightOrigin of the pinning forceA boundary is an imperfection in the crystal structure and as such is associated with a certain quantity of energy. When a boundary passes through an incoherent particle then the portion of boundary that would be inside the particle essentially ceases to exist. In order to move past the particle some new boundary must be created, and this is energetically unfavourable. While the region of boundary near the particle is pinned the rest of the boundary continues trying to move forward under its own driving force. This results in the boundary becoming bowed between those points where it is anchored to the particles. Mathematical descriptionThe figure illustrates a boundary of energy γ per unit area where it intersects with an incoherent particle of radius r. The pinning force acts along the line of contact between the boundary and the particle i.e. a circle of diameter AB = 2πr cosθ. The force per unit length of boundary in contact is γ sinθ. Hence the total force acting on the particle-boundary interface is The maximum restraining force occurs when β = 45° and so Fmax = πrγ . In order to determine the pinning force by a given dispersion of particles Zener made several important assumptions:
For a volume fraction Fv of randomly distributed spherical particles of radius r, the number per unit volume (number density) is given by From this total number density only those particles that are within one particle radius will be able to interact with the boundary. If the boundary is essentially planar then this fraction will be given by Given the assumption that all particles apply the maximum pinning force, Fmax, the total pinning pressure exerted by the particle distribution per unit area of the boundary is This is referred to as the Zener pinning pressure. It follows that large pinning pressures are produced by:
The Zener pinning pressure is orientation dependent, which means that the exact pinning pressure depends on the amount of coherence at the grain boundaries. Notes
- "Contribution à l'étude de la dynamique du zener pinning: simulations numériques par éléments finis", Thesis in French (2003). by G. Couturier. |
| This article is licensed under the GNU Free Documentation License. It uses material from the Wikipedia article "Zener_pinning". A list of authors is available in Wikipedia. |