To use all functions of this page, please activate cookies in your browser.
my.chemeurope.com
With an accout for my.chemeurope.com you can always see everything at a glance – and you can configure your own website and individual newsletter.
- My watch list
- My saved searches
- My saved topics
- My newsletter
1,5-Cyclooctadiene
1,5-Cyclooctadiene, (abb. COD or 1,5-COD), is the chemical compound with the chemical formula C8H12. It is an important ligand in organometallic chemistry.[1][2] Additional recommended knowledge
Synthesis1,5-Cyclooctadiene can be prepared by dimerization of 1-butene in the presence of a nickel catalyst. Reactions1,5-COD binds to metals in as a bis η2 ligand, meaning that it is attached to the metal center via both alkene groups. Complexes of the form M(cod)2, where M is Ni, Pd, or Pt (see image below), are often used as starting materials for the synthesis of these complexes. Metal-COD complexes are attractive because they are sufficiently stable to be isolated but the COD ligands are easily displaced by other ligands. These complexes are more stable than related ethylene complexes. The stability of COD complexes is attributable to the chelate effect.
Synthesis M(COD)2The complex Ni(COD)2 is prepared by reduction of anhydrous nickel acetylacetonate in the presence of the ligand, using triethylaluminium [3]
The related complex, Pt(COD)2, is prepared by a more circuitous route involving the dilithium cyclooctatetraene:[4]
Uses of M(COD)2Extensive work has been reported on complexes of COD, much of which can has been described in Inorganic Syntheses volumes 25, 26 and 28. The platinum complex has been used in many syntheses:
COD complexes are useful as starting materials, one noteworthy example is the reaction:
The product Ni(CO)4 is highly toxic, thus it is advantageous to generate it in the reaction vessel as opposed to being dispensed directly. Complexes with other metalsMany low-valent metals form stable complexes with COD, examples being Mo(COD)(CO)4, [RuCl2(COD)]n, and Fe(COD)(CO)3. COD is an especially important in the coordination chemistry of rhodium(I) and iridium(I), examples being Crabtree's catalyst and cyclooctadiene rhodium chloride dimer. The square planar complexes [M(COD)2]+ are known (M = Rh, Ir). Other reactionsCOD reacts with borane to 9-Borabicyclo[3.3.1]nonane, which is an important reagent in organic chemistry for hydroborations. COD forms in a two-step reaction with first disulfur dichloride and then sulfuryl chloride the compound 2,6-dichloro-9-thiabicyclo[3.3.1]nonane[5] which can be further modified as the di-azide or di-cyano derivative in a nucleophilic substitution aided by anchimeric assistance. References
|
|||||||||||||||||||||||||||
| This article is licensed under the GNU Free Documentation License. It uses material from the Wikipedia article "1,5-Cyclooctadiene". A list of authors is available in Wikipedia. | |||||||||||||||||||||||||||





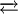 Ni(CO)4 + 2 COD
Ni(CO)4 + 2 COD


