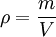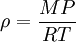To use all functions of this page, please activate cookies in your browser.
my.chemeurope.com
With an accout for my.chemeurope.com you can always see everything at a glance – and you can configure your own website and individual newsletter.
- My watch list
- My saved searches
- My saved topics
- My newsletter
Density
In the common case of a homogeneous substance, density is expressed as: where, in SI Units:
In some cases the density is expressed as a specific gravity or relative or specific density, in which case it is expressed in multiples of the density of some other standard material, usually water or air. Product highlight
HistoryIn a famous problem, Archimedes was given the task of determining whether King Hiero's goldsmith was embezzling gold during the manufacture of a wreath dedicated to the gods and replacing it with another, cheaper alloy.[1] Archimedes knew that the irregular shaped wreath could be smashed into a cube or sphere, where the volume could be calculated more easily when compared with the weight; the king did not approve of this. Baffled, Archimedes went to take a bath and observed from the rise of the water upon entering that he could calculate the volume of the crown through the displacement of the water. Allegedly, upon this discovery, Archimedes went running though the streets in the nude shouting, "Eureka! Eureka!" (Greek "I have found it"). As a result, the term "eureka" entered common parlance and is used today to indicate a moment of enlightenment. This story first appeared in written form in Vitruvius' books of architecture, two centuries after it supposedly took place.[2] Some scholars have doubted the accuracy of this tale, saying among other things that the method would have required precise measurements that would have been difficult to make at the time.[3] Measurement of densityFor a homogeneous object, the formula mass/volume may be used. The mass is normally measured with an appropriate scale; the volume may be measured directly (from the geometry of the object) or by the displacement of a liquid. A very common instrument for the direct measurement of the density of a liquid is the hydrometer. A less common device for measuring fluid density is a pycnometer, a similar device for measuring the absolute density of a solid is a gas pycnometer. Another possibility for determining the density of a liquid or a gas is the measurement with a digital density meter - based on the oscillating U-tube principle. The density of a solid material can be ambiguous, depending on exactly how it is defined, and this may cause confusion in measurement. A common example is sand: if gently filled into a container, the density will be small; when the same sand is compacted into the same container, it will occupy less volume and consequently carry a greater density. This is because "sand" contains a lot of air space in between individual grains; this overall density is called the bulk density, which differs significantly from the density of an individual grain of sand. Common unitsIn U.S. customary units or Imperial units, the units of density include:
Changes of densityIn general density can be changed by changing either the pressure or the temperature. Increasing the pressure will always increase the density of a material. Increasing the temperature generally decreases the density, but there are notable exceptions to this generalisation. For example, the density of water increases between its melting point at 0 °C and 4 °C and similar behaviour is observed in silicon at low temperatures. The effect of pressure and temperature on the densities of liquids and solids is small so that a typical compressibility for a liquid or solid is 10–6 bar–1 (1 bar=0.1 MPa) and a typical thermal expansivity is 10–5 K–1. In contrast, the density of gases is strongly affected by pressure. Boyle's law says that the density of an ideal gas is given by where R is the universal gas constant, P is the pressure, M the molar mass, and T the absolute temperature. This means that a gas at 300 K and 1 bar will have its density doubled by increasing the pressure to 2 bar or by reducing the temperature to 150 K. Density of water
Density of air
References
Books
See also
Categories: Continuum mechanics | Physical chemistry |
|||||||||||||||||||||||||||||||||||||||||||||||||||
| This article is licensed under the GNU Free Documentation License. It uses material from the Wikipedia article "Density". A list of authors is available in Wikipedia. | |||||||||||||||||||||||||||||||||||||||||||||||||||