To use all functions of this page, please activate cookies in your browser.
my.chemeurope.com
With an accout for my.chemeurope.com you can always see everything at a glance – and you can configure your own website and individual newsletter.
- My watch list
- My saved searches
- My saved topics
- My newsletter
Compressibility
In thermodynamics and fluid mechanics, compressibility is a measure of the relative volume change of a fluid or solid as a response to a pressure (or mean stress) change. where V is volume and p is pressure. The above statement is incomplete, because for any object or system the magnitude of the compressibility depends strongly on whether the process is adiabatic or isothermal. Accordingly we define the isothermal compressibility as: where the subscript T indicates that the partial differential is to be taken at constant temperature. The adiabatic compressibility as: where S is entropy. For a solid, the distinction between the two is usually negligible. The inverse of the compressibility is called the bulk modulus, often denoted K (sometimes B). That page also contains some examples for different materials. Product highlight
Fluid DynamicsAeronautical dynamicsCompressibility is an important factor in aerodynamics. At low speeds, the compressibility of air is not significant in relation to aircraft design, but as the airflow nears and exceeds the speed of sound, a host of new aerodynamic effects become important in the design of aircraft. These effects, often several of them at a time, made it very difficult for World War II era aircraft to reach speeds much beyond 800 km/h (500mph). Some of the minor effects include changes to the airflow that lead to problems in control. For instance, the P-38 Lightning with its thick high-lift wing had a particular problem in high-speed dives that led to a nose-down condition. Pilots would enter dives, and then find that they could no longer control the plane, which continued to nose over until it crashed. Adding a "dive flap" beneath the wing altered the center of pressure distribution so that the wing would not lose its lift. This fixed the problem.[1] A similar problem affected some models of the Supermarine Spitfire. At high speeds the ailerons could apply more torque than the Spitfire's thin wings could handle, and the entire wing would twist in the opposite direction. This meant that the plane would roll in the direction opposite to that which the pilot intended, and led to a number of accidents. Earlier models weren't fast enough for this to be a problem, and so it wasn't noticed until later model Spitfires like the Mk.IX started to appear. This was mitigated by adding considerable torsional rigidity to the wings, and was wholly cured when the Mk.XIV was introduced. The Messerschmitt Bf 109 and Mitsubishi Zero had the exact opposite problem in which the controls became ineffective. At higher speeds the pilot simply couldn't move the controls because there was too much airflow over the control surfaces. The planes would become difficult to maneuver, and at high enough speeds less maneuverable aircraft could out-turn them. Finally, another common problem that fits into this category is flutter. At some speeds the airflow over the control surfaces will become turbulent, and the controls will start to flutter. If the speed of the fluttering is close to a harmonic of the control's movement, the resonance could break the control off completely. This was a serious problem on the Zero. When problems with poor control at high speed were first encountered, they were addressed by designing a new style of control surface with more power. However this introduced a new resonant mode, and a number of planes were lost before this was discovered. All of these effects are often mentioned in conjunction with the term "compressibility", but in a manner of speaking, they are incorrectly used. From a strictly aerodynamic point of view, the term should refer only to those side-effects arising as a result of the changes in airflow from an incompressible fluid (similar in effect to water) to a compressible fluid (acting as a gas) as the speed of sound is approached. There are two effects in particular, wave drag and critical mach. Wave drag is a sudden rise in drag on the aircraft, caused by air building up in front of it. At lower speeds this air has time to "get out of the way", guided by the air in front of it that is in contact with the aircraft. But at the speed of sound this can no longer happen, and the air which was previously following the streamline around the aircraft now hits it directly. The amount of power needed to overcome this effect is considerable. The critical mach is the speed at which some of the air passing over the aircraft's wing becomes supersonic. At the speed of sound the way that lift is generated changes dramatically, from being dominated by Bernoulli's principle to forces generated by shock waves. Since the air on the top of the wing is traveling faster than on the bottom, due to Bernoulli effect, at speeds close to the speed of sound the air on the top of the wing will be accelerated to supersonic. When this happens the distribution of lift changes dramatically, typically causing a powerful nose-down trim. Since the aircraft normally approached these speeds only in a dive, pilots would report the aircraft attempting to nose over into the ground. ThermodynamicsThe term "compressibility" is also used in thermodynamics to describe the deviance in the thermodynamic properties of a real gas from those expected from an ideal gas. The compressibility factor is defined as where p is the pressure of the gas, T is its temperature, and Z can, in general, be either greater or less than unity for a real gas. The deviation from ideal gas behavior tends to become particularly significant (or, equivalently, the compressibility factor strays far from unity) near the critical point, or in the case of high pressure or low temperature. In these cases, a generalized Compressibility chart or an alternative equation of state better suited to the problem must be utilized to produce accurate results. Earth sciences
Compressibility is used in the Earth sciences to quantify the ability of a soil or rock to reduce in volume with applied pressure. This concept is important for specific storage, when estimating groundwater reserves in confined aquifers. Geologic materials are made up of two portions: solids and voids (or same as porosity). The void space can be full of liquid or gas. Geologic materials reduces in volume only when the void spaces are reduced, which expel the liquid or gas from the voids. This can happen over a period of time, resulting in settlement. It is an important concept in geotechnical engineering in the design of certain structural foundations. For example, the construction of high-rise structures over underlying layers of highly compressible bay mud poses a considerable design constraint, and often leads to use of driven piles or other innovative techniques. References
See also
Categories: Thermodynamics | Fluid dynamics |
|||||||||||||||||||||||||||||||
| This article is licensed under the GNU Free Documentation License. It uses material from the Wikipedia article "Compressibility". A list of authors is available in Wikipedia. | |||||||||||||||||||||||||||||||


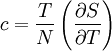
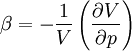
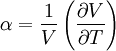
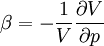
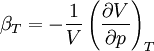
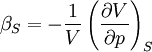



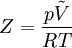
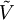 is its
is its