To use all functions of this page, please activate cookies in your browser.
my.chemeurope.com
With an accout for my.chemeurope.com you can always see everything at a glance – and you can configure your own website and individual newsletter.
- My watch list
- My saved searches
- My saved topics
- My newsletter
Electron liquidThe Electron Liquid is a model system used by physicists to theoretically study interactions among electrons. The uniform electron liquid is a well known model for simple metals like sodium or Aluminum. The ionic charges are assumed to be smudged out to form a uniform static positive background, and the electrons are assumed to move in this positive background which maintains charge neutrality. This is also known as the Jellium model. If the Coulomb interaction between the electrons is neglected, then we have the free-electron gas, or the free Fermi gas. However, when the Coulomb interaction is switched on, we have a many-body problem of interacting particles. Additional recommended knowledgeThe weakly interacting regime of densities is known as the electron gas. At zero temperature, this happens if the density is very high. If the number of particles per unit volume (in 3D, or per unit area in 2D) is n, then it is customary to define the radius of the sphere (or disc in 2D) which contains one particle. This radius is denoted by rs, and is known as the Wigner-Seitz radius. In 3-D, rs = (3 / (4πn))1 / 3. It is clear that at high density rs tends to zero. The kinetic energy goes as When rs becomes greater than unity, interactions are important and this regime is known as the electron liquid. The electron liquid model holds up to rs ~ 110 in 3D, and up to rs ~ 35 in 2D. For larger values of rs (i.e, in low-density electron systems), the electron liquid crystallizes forming a crystalline phase known as the Wigner crystal. Perturbation theory fails for electron liquids where rs > 1. Most common metals are in the regime 2 < rs < 6 and hence the metallic regime is poorly described by perturbation theory. However, because of the screening of the Coulomb interaction by other electrons (thus weakening it), a free-electron-like model known as the Landau Fermi-Liquid theory works to some extent. The simplest approximation to the screening of the Coulomb interaction 1 / r by the other electrons is given by the form exp( − r / rTF) / r, where rTF is known as the Thomas-Fermi screening length. At high temperatures, this becomes the Debye-Hukel length. A proper discussion of strongly interacting regimes require the use of methods like: (a) Quantum Monte Carlo simulations. (b) Integral Equation methods, e.g., the CHNC, an acronym for the classical-map hyper-netted-chain method, or the Fermi hyper-netted-chain method. Because of Fermi statistics, the electrons in the electron liquid fill up to an energy level known as the Fermi energy EF. For a metal like Aluminum, EF is of the order of 12 electron Volts. If the temperature is greater than 12 eV, the electron liquid becomes partially degenerate, since the states above the Fermi energy begin to get occupied. Such electron fluids can be used to model dense, finite-temperature plasmas. At very high temperatures, the Debye Hukel classical plasma methods can be used. However, for general strongly interacting plasmas at finite temperatures, other methods based on, say, density functional theory, are needed. References
Plasmas and finite-T electron liquids
Categories: Condensed matter physics | Electron |
| This article is licensed under the GNU Free Documentation License. It uses material from the Wikipedia article "Electron_liquid". A list of authors is available in Wikipedia. |





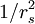 , in atomic units, at T=0. The Coulomb energy goes as
, in atomic units, at T=0. The Coulomb energy goes as 

