Tailored Polymers from a Printer
Efficient chain transfer for 3D-printing of tough photopolymers
An ever-growing number of coatings, including varnishes and printing inks, as well as tooth fillings, are cured with light. Yet, homogenous, tailored, polymer networks cannot be produced, and the materials tend to be brittle, which limits the use of photopolymers in applications like 3D printing, biomedicine, and microelectronics. Researchers present a method by which methacrylate-based, homogenously crosslinked, tailored, tough polymers can be made – even at high resolution for 3D printing.
© Wiley-VCH
Light-curing is usually a radical chain polymerization. An initiator is split into radicals by light energy. These then attack the monomer, such as the C=C double bond in a vinyl group, which forms a new radical that becomes starting point of a growing polymer network by attacking more monomers and binding to them.
Newer methods to better control radical photopolymerization and the material properties of the products tend to slow the curing process, which is not ideal for 3D printing. A short irradiation phase is critical for high spatial resolution and economical production times.
A new approach for the tailored production of methacrylate-based photopolymers without inhibiting the curing process has been developed by a team led by Robert Liska at the Technical University of Vienna (Austria). Their success relies on the addition of an ester-activated vinyl sulfonate ester (EVS), which acts as a chain transfer agent. It is activated because it easily splits off one portion of itself.
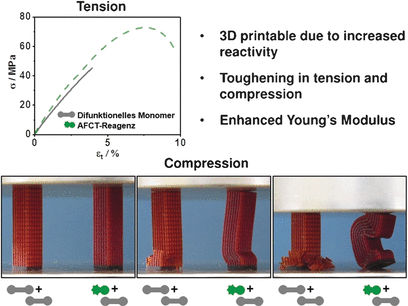
If the growing polymer network attacks EVS instead of the next monomer, an intermediate forms and quickly splits apart to form a terminated polymer chain in the network and a highly reactive radical (tosyl radical), which starts a new chain reaction. The more EVS is added, the shorter the average chain length in the polymer network. Because shorter polymer chains remain mobile longer, the danger of shrinkage cracks during curing is significantly reduced. In contrast to conventional chain transfer agents, the polymerization is not inhibited, because there are no stable intermediates or reversible reaction steps involved. The splitting off of the tosyl radical is favored.
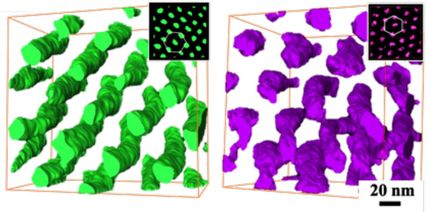
The researchers prepared a scaffold-like sample structure using a methacrylate copolymer. Individual layers with a thickness of 50 µm were spatially well resolved. The material is very homogenous, solid but elastic and impact resistant with high tensile strength. These properties can be adjusted by changing the amount of EVS added. Without EVS, the material was very brittle. This new approach prepares the way for tough, photopolymers for applications in biomedicine, such as shape-memory polymers for tissue growth and as fillings for teeth.
Original publication
Konstanze Seidler et al.; "Vinylsulfonatester: Effiziente Kettenübertragungsreagenzien für verzögerungsfreien 3D‐Druck schlagzäher Photopolymere";
Konstanze Seidler et al.; "Vinyl Sulfonate Esters: Efficient Chain Transfer Agents for the 3D Printing of Tough Photopolymers without Retardation"; Angew. Chem. Int. Ed.; 2018
Most read news
Original publication
Konstanze Seidler et al.; "Vinylsulfonatester: Effiziente Kettenübertragungsreagenzien für verzögerungsfreien 3D‐Druck schlagzäher Photopolymere";
Konstanze Seidler et al.; "Vinyl Sulfonate Esters: Efficient Chain Transfer Agents for the 3D Printing of Tough Photopolymers without Retardation"; Angew. Chem. Int. Ed.; 2018
Topics
Organizations
Other news from the department science

Get the chemical industry in your inbox
From now on, don't miss a thing: Our newsletter for the chemical industry, analytics, lab technology and process engineering brings you up to date every Tuesday and Thursday. The latest industry news, product highlights and innovations - compact and easy to understand in your inbox. Researched by us so you don't have to.