Lefty or righty molecules lend a hand to material structures
Effort to build bottom-up chiral polymers with unique functions
Advertisement
As below, so above. That seems to be an operating principle for molecules that start with a basic chirality - or "handed-ness" -- and pass it on as they combine into larger structures.
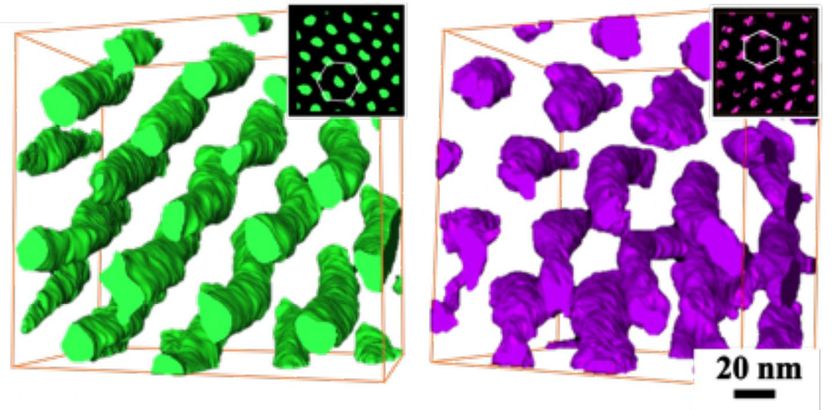
A reconstruction of block copolymers as seen through an electron microscope clearly shows how they follow the chirality, or handedness, established by their basic molecules and grow into spiraling structures that twist left or right. Their controllable 'handed-ness' and tunability could lead to materials with unique optical qualities.
Rong-Ming Ho/ National Tsing Hua University
An international team that included Rice University materials scientist Edwin Thomas pried a new, fundamental detail from its bottom-up creation of several block copolymers, synthetic materials that naturally assemble themselves from small building blocks.
They found the left or right chirality established by the smallest building blocks (monomers) of the polymer replicated itself as the microscopic material came together to form larger scale spiraling structures akin to those commonly found in nature -- for instance, in helical DNA -- and could enable the creation of materials with unique properties.
"From a properties standpoint, chirality is pretty big for optics," Thomas said. "The hope is that we can control self-assembly of chiral entities to make super-chiral entities 10 or 100 times bigger so that they are able to interact with visible or even infrared light."
The discovery led by the Rice professor's experimental colleagues in Taiwan is the focus of a paper in the Proceedings of the National Academy of Sciences.
Thomas and his team have spent years developing expertise in block copolymers, a class of metamaterials that can assemble themselves into many distinct patterns, including alternating layers. One alternating layer copolymer they created proved able to absorb the energy of a micro-scale bullet, while another formed a color-changing film able to act as sensor in food packaging that could detect spoilage and another that could be used to reversibly write in color on ordinary paper.
Thomas noted the importance of chirality in nature, especially in drug design, where a left-handed molecule can be a savior while the same molecule, but right-handed, is toxic. Chiral-specific copolymers that mimic nature could also become tough-but-flexible compounds with unique, tunable properties, he said.
Scientists at the National Tsing Hua and National Chung Cheng universities in Taiwan grew arrays of polymer cylinders from monomers and showed through tomographic electron microscope 3D reconstructions and videos that the cylinders twisted themselves to the left or right as dictated by the molecular building blocks.
Thomas said the resulting elastic polymers could be stretched and tuned on demand to react to specific wavelengths of light. "We could make photonic crystals that reflect right-handed light and transmit left-handed light," he said. "With circularly polarized light, it could transmit for one handedness and reflect for the other handedness. It would be a mirror for right and perfectly transparent for left.
"I'm looking forward to experimenting with light with these materials, because light is fascinating," Thomas said. "You can do things you can literally see by manipulating the material."
He looks forward to the creation of chiral objects that are even more complex. "What if we can make left-chiral structures that fuse into right-chiral structures? And suppose we can do it in three dimensions? What happens there?
"Every time we solve something or think we've found some interesting thing, all we've done is opened up a thousand new questions," he said. "And I have new questions."
Other news from the department science
These products might interest you
Most read news
More news from our other portals
See the theme worlds for related content
Topic world Synthesis
Chemical synthesis is at the heart of modern chemistry and enables the targeted production of molecules with specific properties. By combining starting materials in defined reaction conditions, chemists can create a wide range of compounds, from simple molecules to complex active ingredients.

Topic world Synthesis
Chemical synthesis is at the heart of modern chemistry and enables the targeted production of molecules with specific properties. By combining starting materials in defined reaction conditions, chemists can create a wide range of compounds, from simple molecules to complex active ingredients.